Abstract
Purpose. To study the effect of dose and food on the bioavailability of saquinavir in dogs.
Methods. A Youden Square block design was used for six female mongrel dogs (20-24 kg) who received six saquinavir treatments. The six randomized treatments were 1 mg/kg intravenous infusion over 30 min; 200, 400, 600, and 800 mg of saquinavir in the form of 200-mg capsules given orally with food; and 400 mg of saquinavir given orally after an overnight fast. A 200-mg 14C-saquinavir capsule was used to replace one of the 200-mg unlabeled saquinavir capsules in the 200- and 800-mg oral study.
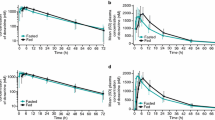
Results. Absorption of saquinavir from the gut was variable. (FA: 49-95%). The 14C-saquinavir study shows that the total radioactivity absorbed from the gut was insignificantly different from that of unlabeled saquinavir, suggesting first-pass gut metabolism was unimportant. The bioavailability of saquinavir under fasting condition was significantly lower (8.41 ± 4.7% vs. 20.3 ± 2.6%, p < 0.05). Saquinavir underwent significant first-pass liver metabolism because hepatic clearance values (22 to 30 ml min-1kg-1) approached that of liver blood flow.
Conclusions. Incomplete gut absorption and extensive first-pass liver metabolism are the causes for low bioavailability of saquinavir in dogs. Absorption was further reduced under fasted conditions.
Similar content being viewed by others
References
M. E. Fitzsimmons and J. M. Collins. Selective biotransformation of the human immunodeficiency virus protease inhibitor saquinavir by human small-intestinal cytochrome P4503A4. Drug Metab. Dispos. 25:256-266 (1997).
V. S. Kitchen, C. Skinner, K. Ariyoshi, E. A. Lane, I. B. Duncan, J. Burckhardt, H. U. Burger, K. Bragman, A. J. Pinching, and J. N. Weber. Safety and activity of saquinavir in HIV infection. Lancet 345:952-954 (1995).
S. Noble and D. Faulds. Saquinavir: A review of its pharmacology and clinical potential in the management of HIV infection. Drugs 52:93-112 (1996).
C. Merry, M. G. Barry, F. Mulcahy, M. Ryan, J. Heavey, J. F. Tjia, S. E. Gibbons, A. M. Breckenridge, and D. J. Back. Saquinavir pharmacokinetics alone and in combination with ritonavir in HIV-infected patients. AIDS 11:F29-F33 (1997).
M. Boffito, P. Carriero, L. Trentini, R. Raiteri, S. Bonora, A. Sinicco, H. E. Reynolds, P. G. Hoggard, D. J. Back, and G. Di Perri. Pharmacokinetics of saquinavir co-administered with cimetidine. J. Antimicrob. Chemother. 50:1081-1084 (2002).
W. Cameron, E. Sun, M. Markowitz, C. Farthing, D. McMahon, D. Poretz, C. Cohen, S. Follansbee, J. H. Mellors, A. Hsu, G. F. Granneman, R. Maki, M. Salgo, J. Court, and J. Leonard. Combination use of ritonavir and saquinavir in HIV-infected patients: preliminary safety and activity data. In: Program and Abstracts of the XI International Conference on AIDS: July 7-12, 1996; Vancouver, British Columbia, Canada. Abstract THB 934.
D. W. O'Brien, H. A. Semple, G. D. Molnar, Y. K. Tam, R. T. Coutts, R. V. Rajotte, and J. Bayens-Simmonds. A chronic conscious dog model for direct transhepatic studies in normal and pancreatic islet cell transplanted dogs. J. Pharmacol. Methods 25:157-170 (1991).
H. R. Wiltshire, K. J. Prior, J. Dhesi, F. Trach, M. Schlageter, and H. Schöenberger. The synthesis of labeled forms of saquinavir. J. Labelled Cpd. Radiopharm. 41:1103-1126 (1998).
N. G. Knebel, S. R. Sharp, and M. J. Madigan. Quantification of the anti-HIV drug saquinavir by high-speed on-line high performance liquid chromatography/tandem mass spectrometry. J. Mass Spectrometry 30:1149-1156 (1995).
C. Ediss and Y. K. Tam. An interactive computer program for determining areas bounded by drug concentration curves using Lagrange interpolation. J. Pharmacol. Toxicol. Methods 34:165-168 (1995).
H. A. Semple, Y. K. Tam, and R. T. Coutts. Hydralazine pharmacokinetics and interaction with food: an evaluation of the dog as an animal model. Pharm. Res. 7:274-279 (1990).
A. Skerjanec, S. Tawfik, and Y. K. Tam. Nonlinear pharmacokinetics of mibefradil in the dog. J. Pharm. Sci. 85:189-192 (1996).
J. Alsenz, H. Steffen, and R. Alex. Active apical secretory efflux of the HIV protease inhibitors saquinavir and ritonavir in Caco-2 cell monolayers. Pharm. Res. 15:423-428 (1998).
C. B. Washington, H. R. Wiltshire, M. M. T. Moy, S. R. Harris, E. W. D. Hall, L. M. P. Weigl, Z. Liang, and T. F. Blaschke. The disposition of saquinavir in normal and P-glycoprotein deficient mice and in cultured cells. Drug Metab. Dispos. 28:1058-1062 (2000).
M. T. Huisman, J. W. Smit, H. R. Wiltshire, J. H. Beijnen, and A. H. Schinkel. Assessing safety and efficacy of directed P-glycoprotein inhibition to improve the pharmacokinetic properties of saquinavir coadministered with ritonavir. J. Pharmacol. Ther. 304:596-602 (2003).
V. D. Makhey, A. Guo, D. A. Norris, and P. Hu. Characterization of the regional intestinal kinetics of drug efflux in rat and human intestine and in Caco-2 cells. Pharm. Res. 15:1160-1167 (1998).
M. F. Paine, M. Khalighi, J. M. Fisher, D. D. Shen, K. L. Kunze, C. L. Marsh, J. D. Perkins, and K. E. Thummel. Characterization of interintestinal and intraintestinal variations in human CYP3A-dependent metabolism. J. Pharmacol. Exper. Ther. 283:1552-1562 (1997).
C. M. Perry and S. Noble. Saquinavir: soft-gel capsule formulation. A review of its use in patients with HIV infection. Drugs 55:461-486 (1998).
V. A. Eagling, H. Wiltshire, I. W. A. Whitcombe, and D. J. Back.C. YP3A4 mediated hepatic metabolism of the HIV-1 protease inhibitor saquinavir in-vitro. Xenobiotica 32:1-17 (2002).
H. H. T. Kupferschmidt, K. E. Fattinger, H. R. Ha, F. Follac, and S. Krädahenbühl. Grapefruit juice enhances the bioavailability of the HIV protease inhibitor saquinavir in man. Br. J. Clin. Pharmacol. 45:355-359 (1998).
K. S. Lown, D. G. Bailey, R. J. Fontana, S. K. Janardan, C. A. Adair, L. A. Fortiage, M. B. Brown, W. Guo, and P. B. Watkins. Grapefruit juice increases felodopine oral availability in humans by decreasing intestinal CYP3A protein expression. J. Clin. Invest. 99:2545-2553 (1997).
J. L. Steimer, B. Fotteler, R. Gieschke, H. Wiltshire, and N. Buss. Predicting the optimal dose of saquinavir via modelling of dose-exposure and exposure-effect relationships. In measurement and kinetics of in vivo drug effects. In M. Danhof and J. L. Steimer (eds.), Advances in Simultaneous Pharmacokinetic/Pharmacodynamic Modelling. Center for Drug Research, Leiden/Amsterdam, 1998, pp. 79-86.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tam-Zaman, N., Tam, Y.K., Tawfik, S. et al. Factors Responsible for the Variability of Saquinavir Absorption: Studies Using an Instrumented Dog Model. Pharm Res 21, 436–442 (2004). https://doi.org/10.1023/B:PHAM.0000019296.47762.3f
Issue Date:
DOI: https://doi.org/10.1023/B:PHAM.0000019296.47762.3f