Abstract
Purpose. To study the reaction of a series of Hantzsch dihydropyridines with pharmacological significance such as, nifedipine, nitrendipine, nisoldipine, nimodipine, isradipine and felodipine, with electrogenerated superoxide in order to identify products and postulate a mechanism.
Methods. The final pyridine derivatives were separated and identified by gas chromatography/mass spectrometry (GC-MS). The intermediates, anion dihydropyridine and the HO2 •/ HO2 − species, were observed from voltammetric studies and controlled potential electrolysis was used to electrogenerate O2 • −.
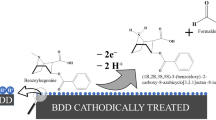
Results. The current work reveals that electrogenerated superoxide can quantitatively oxidize Hantzsch dihydropyridines to produce the corresponding aromatized pyridine derivatives.
Conclusions. Our results indicate that the aromatization of Hantzsch dihydropyridines by superoxide is initiated by proton transfer from the N1-position on the 1,4-dihydropyridine ring to give the corresponding anion dihydropyridine, which readily undergoes further homogeneous oxidations to provide the final aromatized products. The oxidation of the anionic species of the dihydropyridine is more easily oxidized than the parent compound.
Similar content being viewed by others
References
B. Halliwell and J. M. C. Gutteridge. Free Radicals in Biology and Medicine, 3rd ed., University Press, New York, 1999.
D. T. Sawyer, M. J. Gibian, M. M. Morrison, and E. T. Sea. On the chemical reactivity of superoxide. J. Am. Chem. Soc. 100:627-628 (1978).
T. Okajima and T. Osaka. Chemiluminiscence of 3-methylindole based on electrogeneration of superoxide ion in acetonitrile solutions. J. Electroanal. Chem. 523:34-39 (2002).
M. V. Merrit and D. T. Sawyer. Electrochemical studies of the reactivity of superoxide ion with several alkyl halides in dimethyl sulfoxide. J. Org. Chem. 35:2157-2159 (1970).
D. T. Sawyer, J. J. Stamp, and K. A. Menton. Reactivity of superoxide ion with ethyl pyruvate, α-diketones, and benzil in dimethylformamide. J. Org. Chem. 48:3733-3736 (1983).
R. Dietz, A. E. J. Forno, B. E Larcombe., and M. E. Peover. Nucleophilic reactions of electrogenerated superoxide ion. J. Chem. Soc. B. 1970; 816-820.
T. S. Calderwood, R. C. Neuman, and D. T. Sawyer. Oxygenation of chloroalkenes by superoxide in aprotic media. J. Am. Chem. Soc. 105:3337-3339 (1983).
R. Poupko and I. Rosenthal. Electron transfer interactions between superoxide ion and organic compounds. J. Phys. Chem. 77:1722-1724 (1973).
J. S. Valentine and A. B. Curtis. A convenient preparation of solutions of superoxide anion and the reaction of superoxide anion with a copper(II) complex. J. Am. Chem. Soc. 97:224-226 (1975).
Y. Moro-oka and C. S. Foote. Chemistry of superoxide ion. I. Oxidation of 3,5-di-tert-butylcatechol with potassium superoxide. J. Am. Chem. Soc. 98:1510-1514 (1976).
D. T. Sawyer and M. J. Gibian. The chemistry of superoxide ion. Tetrahedron 35:1471-1481 (1979).
O. Aruoma, C. Smith, R. Cecchini, P. Evans, and B. Halliwell. Free radical scavenging and inhibition of lipid peroxidation by β-blockers and by agents that interfere with calcium metabolism: a physiologically-significant process? Biochem. Pharm. 42:735-743 (1991).
R. P. Mason, I. T. Mak, M. W. Trumbore, and P. E. Mason. Antioxidant properties of calcium antagonists related to membrane biophysical interactions. Am. J. Cardiol. 84:16L-22L (1999).
I. Mak, P. Boheme, and W. Weglicki. Antioxidant effects of calcium channel blockers against free radical injury in endothelial cells. Circ. Res. 70:1099-1103 (1992).
F. Van Amsterdam, A. Roveri, M. Maiorino, E. Ratti, and F. Ursini. Lacidipine: a dihydropyridine calcium antagonist with antioxidant activity. Free Rad. Biol. Med. 12:183-187 (1992).
R. Toniolo, F. Di Narda, G. Bontempelli, and F. Ursini. An electroanalytical investigation on the redox properties of lacidipine supporting its anti-oxidant effect. Bioelectrochem. 51:193-200 (2000).
G. Díaz-Araya, L. Godoy, L. Naranjo, J. A. Squella, M. E. Letelier, and L. J. Núñez-Vergara. Antioxidant effects of 1,4-dihydropyridine and nitroso aryl derivatives on the Fe+3/ascorbate-stimulated lipid peroxidation in rat brain slices. Gen. Pharmac. 31:385-391 (1998).
N. Nakamichi, Y. Kawashita, and M. Hayashi. Oxidative aromatization of 1,3,5-trisubstituted pyrazolines and Hantzsch 1,4-dihydropyridines by Pd/C in acetic acid. Org. Lett. 4:3955-3957 (2002).
J. R. Pfister. Rapid, high-yield oxidation of hantzsch-type 1,4-dihydropyridines with ceric ammonium-nitrate. Synthesis Stuttgart 8:689-690 (1990).
A. Maquestiau, A. Mayence, and J. Eynde. Ultrasound-promoted aromatization of Hantzsch 1,4-dihydropyridines by clay-supported cuptic nitrate. Tetrahedron Lett. 32:3839-3840 (1991).
J. Eynde, A. Mayence, and A. Maquestiau. A novel application of the oxidizing properties of pyridinium chlorochromate: aromatization of Hantzsch 1,4-dihydropyridines. Tetrahedron 48:463-468 (1992).
R. Böcker and F. P. Guengerich. Oxidation of 4-aryl-and 4-alkyl-substituted 2,6-dimethyl-3,5-bis(alkoxycarbonyl)-1,4-dihydropyridines by human liver microsomes and immunochemical evidence for the involvement of a form of cytochrome P-450. J. Med. Chem. 29:1596-1603 (1986).
T. Itoh, K. Nagata, M. Okada, and A. Ohsawa. Tetrahedron Lett. 36:2269-2272 (1995).
X.-Q. Zhu, B.-J. Zhao, and J.-P. J. Cheng. Org. Chem. 65:8158-8163 (2000).
M. E. Ortiz, L. J. Nüñez-Vergara, and J. A. Squella. Cyclic voltammetric behaviour of the O2/O2 ∸-redox couple at HMDE and its interaction with nisoldipine. J.Electroanal.Chem. 519:46-52 (2002).
M. E. Ortiz, L. J. Nüñez-Vergara, and J. A. Squella. Relative reactivity of dihydropyridine derivatives to electrogenerated superoxide ion in DMSO solutions: a voltammetric approach. Pharm. Res. 20:289-293 (2003).
C. López-Alarcón, P. Navarrete, C. Camargo, J. A. Squella, and L. J. Nüñez-Vergara. Reactivity of 1,4-dihydropyridines toward alkyl, alkylperoxyl radicals, and ABTS radical cation. Chem. Res. Toxicol. 16:208-215 (2003).
R. Arudi, O. Allen, and R. Bielski. Some observations on the chemistry of KO2-DMSO solutions. FEBS Lett. 135:265-267 (1981).
J. Zhang, W. Pietro, and A. Lever. Rotating ring-disc electrode analysis of CO2 reduction electrocatalyzed by a cobalt tetramethylpyridoporphyrazine on the disk and detected as CO on platinum ring. J. Electroanal.Chem. 403:93-100 (1996).
R. S. Nicholson. Semiempirical procedure for measuring with stationary electrode polarography rates of chemical reactions involving the product of electron transfer. Anal. Chem. 38:1406(1966).
T. Okajima and T. Ohsaka. Chemiluminescence of indole and its derivatives induced by electrogenerated superoxide ion in acetonitrile solutions. Electrochimica Acta 47:1561-1565 (2002).
D. T. Sawyer, G. Chericato Jr. C. T. Angelis, E. J. Nanni Jr., and T. Tsuchiya. Effects of media and electrode materials on the electrochemical reduction of dioxygen. Anal. Chem. 54:1720-1724 (1982).
J. A. Squella, G. Jiménez, S. Bollo, and L. J. Nüñez-Vergara. Electroreduction of 4-(nitrophenyl) substituted 1,4-dihydropyridines on the mercury electrode in aprotic medium. Electrochimica Acta 42:2305-2312 (1997).
E. J. Nanni, M. D. Stallings, and D. T. Sawyer. Does superoxide ion oxidize catechol, α-tocopherol, and ascorbic acid by direct electron transfer? J. Am. Chem. Soc. 102:4481-4485 (1980).
P. Cofré and D. T. Sawyer. Redox chemistry of hydrogen peroxide in anhydrous acetonitrile. Inorg. Chem. 25:2089-2092 (1986).
P. S. Prabha, U. N. Das, R. Koratkar, P. Sangeetha Sagar, and G. Ramesh. Free radical generation, lipid peroxidation and essential fatty acid in uncontrolled essential hypertension, Prostaglandine. Leukotrienes Essent. Fatty Acids 41:23-27 (1990).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ortiz, M.E., Núñez-Vergara, L.J., Camargo, C. et al. Oxidation of Hantzsch 1,4-Dihydropyridines of Pharmacological Significance by Electrogenerated Superoxide. Pharm Res 21, 428–435 (2004). https://doi.org/10.1023/B:PHAM.0000019295.32103.e4
Issue Date:
DOI: https://doi.org/10.1023/B:PHAM.0000019295.32103.e4