Abstract
Purpose. The purpose of this study was (i) to develop glancing angle x-ray powder diffractometry (XRD) as a method for profiling phase transformations as a function of tablet depth; and (ii) to apply this technique to (a) study indomethacin crystallization during dissolution of partially amorphous indomethacin tablets and to (b) profile anhydrate → hydrate transformations during dissolution of theophylline tablets.
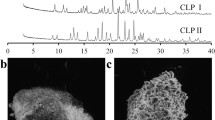
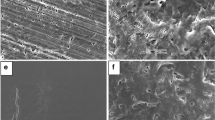
Methods. The intrinsic dissolution rates of indomethacin and theophylline were determined after different pharmaceutical processing steps. Phase transformations during dissolution were evaluated by various techniques. Transformation in the bulk and on the tablet surface was characterized by conventional XRD and scanning electron microscopy, respectively. Glancing angle XRD enabled us to profile these transformations as a function of depth from the tablet surface.
Results. Pharmaceutical processing resulted in a decrease in crystallinity of both indomethacin and theophylline. When placed in contact with the dissolution medium, while indomethacin recrystallized, theophylline anhydrate rapidly converted to theophylline monohydrate. Due to intimate contact with the dissolution medium, drug transformation occurred to a greater extent at or near the tablet surface. Glancing angle XRD enabled us to depth profile the extent of phase transformations as a function of the distance from the tablet surface. The processed sample (both indomethacin and theophylline) transformed more rapidly than did the corresponding unprocessed drug. Several challenges associated with the glancing angle technique, that is, the effects of sorbed water, phase transformations during the experimental timescale, and the influence of phase transformation on penetration depth, were addressed.
Conclusions. Increased solubility, and consequently dissolution rate, is one of the potential advantages of metastable phases. This advantage is negated if, during dissolution, the metastable to stable transformation rate ≫ dissolution rate. Glancing angle XRD enabled us to quantify and thereby profile phase transformations as a function of compact depth. The technique has potential utility in monitoring surface reactions, both chemical decomposition and physical transformations, in pharmaceutical systems.
Similar content being viewed by others
REFERENCES
L. Lachman, H. A. Lieberman, and J. L. Kanig. The Theory and Practice of Industrial Pharmacy. Lea & Febiger, Philadelphia, 1986.
A. J. Aguiar, K. C. J. John, A. W. Kinkel, and J. C. Samyn. Effect of polymorphism on the absorption of chloramphenicol from chloramphenicol palmitate. J. Pharm. Sci. 56:847-853 (1967).
K. Kimura, F. Hirayama, and K. Uekama. Characterization of tolbutamide polymorphs (Burger's forms II and IV) and polymorphic transition behavior. J. Pharm. Sci. 88:385-391 (1999).
Y. Kobayashi, S. Ito, S. Itai, and K. Yamamoto. Physicochemical properties and bioavailability of carbamazepine polymorphs and dihydrate. Int. J. Pharm. 193:137-146 (2000).
Federal Register Part V and F. Department of Health and Human Services. International Conference on Harmonization; Draft Guidance on Specifications: Test procedures and Acceptance Criteria for New Drug Substances and New Drug Products: Chemical Substances; Notice, 1997.
Y. Chikaraishi, M. Otsuka, and Y. Matsuda. Dissolution phenomenon of the piretanide amorphous form involving phase change. Chem. Pharm. Bull. 44:2111-2115 (1996).
A. T. Florence and E. G. Salole. Changes in crystallinity and solubility on comminution of digoxin and observations on spironolactone and estradiol. J. Pharm. Pharmacol. 28:637-642 (1976).
Y. Kato and M. Kohketsu. Relationship between polymorphism and bioavailability of amobarbital in the rabbit. Chem. Pharm. Bull. 29:268-272 (1981).
M. Ono, Y. Tozuka, T. Oguchi, S. Yamamura, and K. Yamamoto. Effects of dehydration temperature on water vapor adsorption and dissolution behavior of carbamazepine. Int. J. Pharm. 239:1-12 (2002).
B. C. Hancock and M. Parks. What is the true solubility advantage for amorphous pharmaceuticals? Pharm. Res. 17:397-404 (2000).
H. Vromans, G. K. Bolhuis, C. F. Lerk, and K. D. Kussendrager. Studies on tableting properties of lactose. VIII. The effect of variations in primary particle size, percentage of amorphous lactose and addition of a disintegrant on the disintegration of spray-dried lactose tablets. Int. J. Pharm. 39:201-206 (1987).
M. F. Doerner and S. Brennan. Strain distribution in thin aluminum films using x-ray depth profiling. J. Appl. Phys. 63:126-131 (1988).
B. L. Ballard, X. Zhu, P. K. Predecki, D. Albin, A. Gabor, J. Tuttle, and R. Noufi. Determination of composition and phase depth-profiles in multilayer and gradient solid solution photovoltaic films using grazing incidence X-ray diffraction. Adv. X-Ray Anal. 38:269-276 (1995).
P. Eisenberger and W. C. Marra. X-ray diffraction study of the germanium (001) reconstructed surface. Phys. Rev. Lett. 46:1081-1084 (1981).
L. G. Parratt. Surface studies of solids by total reflection of X-rays. Phys. Rev. 95:359-369 (1954).
X. Zhu, B. Ballard, and P. Predecki. Determination of z-profiles of diffraction data from tau-profiles using a numerical linear inversion method. Adv. X-Ray Anal. 38:255-262 (1995).
T. C. Huang. Surface and ultra-thin film characterization by grazing-incidence asymmetric Bragg diffraction. Adv. X-Ray Anal. 33:91-99 (1990).
B. E. Warren. X-Ray Diffraction. Addison Wesley, Reading, MA, 1969.
M. Otsuka, T. Matsumoto, and N. Kaneniwa. Effect of environmental temperature on polymorphic solid-state transformation of indomethacin during grinding. Chem. Pharm. Bull. 34:1784-1793 (1986).
E. Suzuki, K. Shimomira, and K. Sekiguchi. Thermochemical study of theophylline and its hydrate. Chem. Pharm. Bull. 37:493-497 (1989).
K. R. Morris, U. J. Griesser, C. J. Eckhardt, and J. G. Stowell. Theoretical approaches to physical transformations of active pharmaceutical ingredients during manufacturing processes. Adv. Drug Del. Rev. 48:91-114 (2001).
J. H. Collett, J. A. Rees, and N. A. Dickinson. Some parameters describing the dissolution rate of salicylic acid at controlled pH. J. Pharm. Pharmacol. 24:724-728 (1972).
C. Doherty and P. York. Mechanisms of dissolution of frusemide/PVP solid dispersions. Int. J. Pharm. 34:197-205 (1987).
M. Otsuka and N. Kaneniwa. A kinetic study of the crystallization process of noncrystalline indomethacin under isothermal conditions. Chem. Pharm. Bull. 36:4026-4032 (1988).
M. Yoshioka, B. C. Hancock, and G. Zografi. Crystallization of indomethacin from the amorphous state below and above its glass transition temperature. J. Pharm. Sci. 83:1700-1705 (1994).
V. Andronis, M. Yoshioka, and G. Zografi. Effects of sorbed water on the crystallization of indomethacin from the amorphous state. J. Pharm. Sci. 86:346-351 (1997).
M. O'Brien, J. McCauley, and E. Cohen. Indomethacin. In K. Florey (ed.), Analytical Profiles of Drug Substances, Vol. 13. Academic Press, San Diego, 1984, pp. 211-238.
P. Predecki. Determination of depth profiles from X-ray diffraction data. Powder Diffraction 8:122-126 (1993).
Powder Diffraction File 26-1893. International Centre for Diffraction Data, Newtown Square, PA, 1997.
S. Budaveri, M. J. O'Neil, A. Smith, P. E. Heckelman, and J. F. Kinneary. The Merck Index. Merck & Co., Whitehouse Station, NJ, 1996.
Powder Diffraction File 27-1977. International Centre for Diffraction Data, Newtown Square, PA, 1997.
R. Jenkins. New directions in the X-ray diffraction analysis of organic materials. Adv. X-Ray Anal. 35:653-660 (1991).
T. Ely, P. K. Predecki, and I. C. Noyan. A method for obtaining stress-depth profiles by absorption constrained profile fitting of diffraction peaks. Adv. X-Ray Anal. 43:1-10 (2000).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Debnath, S., Predecki, P. & Suryanarayanan, R. Use of Glancing Angle X-Ray Powder Diffractometry to Depth-Profile Phase Transformations During Dissolution of Indomethacin and Theophylline Tablets. Pharm Res 21, 149–159 (2004). https://doi.org/10.1023/B:PHAM.0000012163.89163.f8
Issue Date:
DOI: https://doi.org/10.1023/B:PHAM.0000012163.89163.f8