Abstract
Purpose. Cancer cells may circumvent the cytotoxic effect of antimetabolite drugs that inhibit de novo nucleotide synthesis via the uptake of extracellular preformed nucleobases or nucleosides. The goal of this study was to investigate the nucleobase transport mechanism in human U-118 glioblastoma cells and to determine whether the purine nucleobase hypoxanthine affects cell susceptibility to methotrexate.
Methods. Uptake experiments were performed using 3H-labeled hypoxanthine. RT-PCR was used to determine the expression of nucleoside transporters. Methotrexate-induced apoptosis was analyzed using annexin V staining and FACScan analysis.
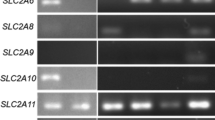
Results. Hypoxanthine transport in U-118 cells involved both carrier-mediated (Km= 10.5 ± 6.3 μM, Vmax= 1.45 ± 0.69 pmol/105 cells/60 s) and simple diffusion processes (Kd= 0.36 ± 0.009 μm/105 cells/60 s). Uptake was sensitive to Na+ and inhibited by nucleobases but not nucleosides or nucleoside transport inhibitors. In contrast, uptake of a nucleoside, uridine, was inhibited by nucleosides but not nucleobases. RT-PCR analysis suggested the presence of hENT1, hENT2, and hCNT1 nucleoside transporters in U-118 cells. In the absence of hypoxanthine, methotrexate inhibited U-118 cell proliferation and induced apoptosis. These toxic effects were diminished when hypoxanthine was present at physiologically relevant concentrations.
Conclusions. Hypoxanthine transport in U-118 cells involves a Na+-dependent, high-affinity nucleobase transport system functionally distinct from nucleoside transporters. At physiologic concentrations, hypoxanthine protects glioblastoma cells from the cytotoxicity of methotrexate.
Similar content being viewed by others
References
D. A. Griffith and S. M. Jarvis. Nucleoside and nucleobase transport systems of mammalian cells. Biochim. Biophys. Acta 1286:153-181 (1996).
S. A. Baldwin, J. R. Mackey, C. E. Cass, and J. D. Young. Nucleoside transporters: molecular biology and implications for therapeutic development. Mol. Med. Today 5:216-224 (1999).
A. R. Kinsella, D. Smith, and M. Pickard. Resistance to chemotherapeutic antimetabolites: a function of salvage pathway involvement and cellular response to DNA damage. Br. J. Cancer 75:935-945 (1997).
M. Fox, J. M. Boyle, and A. R. Kinsella. Nucleoside salvage and resistance to antimetabolite anticancer agents. Br. J. Cancer 64:428-436 (1991).
J. K. Willson, P. H. Fischer, S. C. Remick, K. D. Tutsch, J. L. Grem, L. Nieting, D. Alberti, J. Bruggink, and D. L. Trump. Methotrexate and dipyridamole combination chemotherapy based upon inhibition of nucleoside salvage in humans. Cancer Res. 49:1866-1870 (1989).
R. N. Turner, G. W. Aherne, and N. J. Curtin. Selective potentiation of lometrexol growth inhibition by dipyridamole through cell-specific inhibition of hypoxanthine salvage. Br. J. Cancer 76:1300-1307 (1997).
P. G. Smith, H. D. Thomas, H. C. Barlow, R. J. Griffin, B. T. Golding, A. H. Calvert, D. R. Newell, and N. J. Curtin. In vitro and in vivo properties of novel nucleoside transport inhibitors with improved pharmacological properties that potentiate antifolate activity. Clin. Cancer Res. 7:2105-2113 (2001).
J. Wang, M. E. Schaner, S. Thomassen, S. F. Su, M. Piquette-Miller, and K. M. Giacomini. Functional and molecular characteristics of Na+-dependent nucleoside transporters. Pharm. Res. 14:1524-1532 (1997).
M. W. Ritzel, A. M. Ng, S. Y. Yao, K. Graham, S. K. Loewen, K. M. Smith, R. G. Ritzel, D. A. Mowles, P. Carpenter, X. Z. Chen, E. Karpinski, R. J. Hyde, S. A. Baldwin, C. E. Cass, and J. D. Young. Molecular identification and characterization of novel human and mouse concentrative Na+-nucleoside cotransporter proteins (hCNT3 and mCNT3) broadly selective for purine and pyrimidine nucleosides (system cib). J. Biol. Chem. 276:2914-2927 (2001).
B. A. Domin, W. B. Mahony, and T. P. Zimmerman. Purine nucleobase transport in human erythrocytes. Reinvestigation with a novel ‘inhibitor-stop’ assay. J. Biol. Chem. 263:9276-9284 (1988).
D. A. Griffith and S. M. Jarvis. High affinity sodium-dependent nucleobase transport in cultured renal epithelial cells (LLC-PK1). J. Biol. Chem. 268:20085-20090 (1993).
M. Shayeghi, R. Akerman, and S. M. Jarvis. Nucleobase transport in opossum kidney epithelial cells and Xenopus laevis oocytes: the characterisation, structure-activity relationship of uracil analogues and oocyte expression studies of sodium-dependent and-independent hypoxanthine uptake. Biochim Biophys Acta 1416:109-118 (1999).
C. B. Washington and K. M. Giacomini. Mechanisms of nucleobase transport in rabbit choroid plexus. Evidence for a Na+-dependent nucleobase transporter with broad substrate selectivity. J. Biol. Chem. 270:22816-22819 (1995).
S. Y. Yao, A. M. Ng, M. F. Vickers, M. Sundaram, C. E. Cass, S. A. Baldwin, and J. D. Young. Functional and molecular characterization of nucleobase transport by recombinant human and rat equilibrative nucleoside transporters 1 and 2. Chimeric constructs reveal a role for the ENT2 helix 5-6 region in nucleobase translocation. J. Biol. Chem. 277:24938-24948 (2002).
P. MacDonnell. K. Huff, L. Grouse, and G. Guroff. Brain nucleic acids. In S. Kumar (ed.), Biochemistry of Brain, Pergamon Press, New York, 1980, pp. 211-240.
R. Spector. Hypoxanthine transport and metabolism in the central nervous system. J. Neurochem. 50:969-978 (1988).
M. L. Jimenez, J. G. Puig, F. A. Mateos, T. H. Ramos, I. P. Castroviejo, and J. O. Vazquez. Hypoxanthine and xanthine transport through the blood-brain barrier in hypoxanthine phosphoribosyltransferase (HPRT) deficiency. Adv. Exp. Med. Biol. (1989) 173-179.
Y. Natsumeda, T. Ikegami, E. Olah, and G. Weber. Significance of purine salvage in circumventing the action of antimetabolites in rat hepatoma cells. Cancer Res. 49:88-92 (1989).
E. Marshman, G. A. Taylor, H. D. Thomas, D. R. Newell, and N. J. Curtin. Hypoxanthine transport in human tumour cell lines: relationship to the inhibition of hypoxanthine rescue by dipyridamole. Biochem. Pharmacol. 61:477-484 (2001).
C. H. Rickert and W. Paulus. Epidemiology of central nervous system tumors in childhood and adolescence based on the new WHO classification. Childs Nerv. Syst. 17:503-511 (2001).
I. V. Pech, K. Peterson, and J. G. Cairncross. Chemotherapy for brain tumors. Oncology 12:537-543 (1998).
H. T. Abelson, D. W. Kufe, A. T. Skarin, P. Major, W. Ensminger, G. P. Beardsley, and G. P. Canellos. Treatment of central nervous system tumors with methotrexate. Cancer Treat. Rep. 65(Suppl 1):137-140 (1981).
G. J. Lesser and S. A. Grossman. The chemotherapy of adult primary brain tumors. Cancer Treat. Rev. 19:261-281 (1993).
Y. Iwadate, S. Fujimoto, and A. Yamaura. Differential chemosensitivity in human intracerebral gliomas measured by flow cytometric DNA analysis. Int. J. Mol. Med. 10:187-192 (2002).
J. Wang, S. F. Su, M. J. Dresser, M. E. Schaner, C. B. Washington, and K. M. Giacomini. Na+-dependent purine nucleoside transporter from human kidney: cloning and functional characterization. Am. J. Physiol. 273(6 Pt 2):F1058-F1065 (1997).
I. Vermes, C. Haanen, H. Steffens-Nakken, and C. Reutelingsperger. A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled Annexin V. J. Immunol. Methods 184:39-51 (1995).
C. J. Sinclair, C. G. LaRiviere, J. D. Young, C. E. Cass, S. A. Baldwin, and F. E. Parkinson. Purine uptake and release in rat C6 glioma cells: nucleoside transport and purine metabolism under ATP-depleting conditions. J. Neurochem. 75:1528-1538 (2000).
P. G. Smith, E. Marshman, A. H. Calvert, D. R. Newell, and N. J. Curtin. Prevention of thymidine and hypoxanthine rescue from MTA (LY231514) growth inhibition by dipyridamole in human lung cancer cell lines. Semin. Oncol. 26(2 Suppl 6):63-67 (1999).
L. Genestier, R. Paillot, S. Fournel, C. Ferraro, P. Miossec, and J. P. Revillard. Immunosuppressive properties of methotrexate: apoptosis and clonal deletion of activated peripheral T cells. J. Clin. Invest. 102:322-328 (1998).
C. A. Warlick, C. L. Sweeney, and R. S. McIvor. Maintenance of differential methotrexate toxicity between cells expressing drug-resistant and wild-type dihydrofolate reductase activities in the presence of nucleosides through nucleoside transport inhibition. Biochem. Pharmacol. 59:141-151 (2000).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kong, W., Wang, J. Hypoxanthine Transport in Human Glioblastoma Cells and Effect on Cell Susceptibility to Methotrexate. Pharm Res 20, 1804–1811 (2003). https://doi.org/10.1023/B:PHAM.0000003378.16802.97
Issue Date:
DOI: https://doi.org/10.1023/B:PHAM.0000003378.16802.97