Abstract
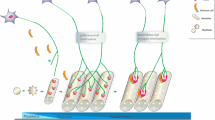
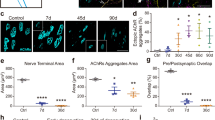
Although physiological differences among neuromuscular junctions (NMJs) have long been known, NMJs have usually been considered as one type of synapse, restricting their potential value as model systems to investigate mechanisms controlling synapse assembly and plasticity. Here we discuss recent evidence that skeletal muscles in the mouse can be subdivided into two previously unrecognized subtypes, designated FaSyn and DeSyn muscles. These muscles differ in the pattern of neuromuscular synaptogenesis during embryonic development. Differences between classes are intrinsic to the muscles, and manifest in the absence of innervation or agrin. The distinct rates of synaptogenesis in the periphery may influence processes of circuit maturation through retrograde signals. While NMJs on FaSyn and DeSyn muscles exhibit a comparable anatomical organization in postnatal mice, treatments that challenge synaptic stability result in nerve sprouting, NMJ remodeling, and ectopic synaptogenesis selectively on DeSyn muscles. This anatomical plasticity of NMJs diminishes greatly between 2 and 6 months postnatally. NMJs lacking this plasticity are lost selectively and very early on in mouse models of motoneuron disease, suggesting that disease-associated motoneuron dysfunction may fail to initiate maintenance processes at “non-plastic” NMJs. Transgenic mice overexpressing growth-promoting proteins in motoneurons exhibit greatly enhanced stimulus-induced sprouting restricted to DeSyn muscles, supporting the notion that anatomical plasticity at the NMJ is primarily controlled by processes in the postsynaptic muscle. The discovery that entire muscles in the mouse differ substantially in the anatomical plasticity of their synapses establishes NMJs as a uniquely advantageous experimental system to investigate mechanisms controlling synaptic rearrangements at defined synapses in vivo.
Similar content being viewed by others
References
AIGNER, L., ARBER, S., KAPFHAMMER, J. P., LAUX, T., SCHNEIDER, C., BOTTERI, F., BRENNER, H. R. & CARONI, P. (1995) Overexpression of the neural growth-associated protein GAP-43 induces nerve sprouting in the adult nervous system of transgenic mice. Cell 83, 26–278.
AKAABOUNE, M., CULICAN, S. M., TURNEY, S. G. & LICHTMAN, J. W. (1999) Rapid and reversible effects of activity on acetylcholine receptor density at the neuromuscular junction in vivo. Science 286, 50–507.
ANTONINI, A., GAGIOLINI, M. & STRYKER, M. P. (1999) Anatomical correlates of functional plasticity in mouse visual cortex. Journal of Neuroscience 19, 438–4406.
APEL, E. D., GLASS, D. J., MOSCOSO, L. M., YANCOPOULOS, G. D. & SANES, J. R. (1997) Rapsyn is required for MuSK signaling and recruits synaptic components to a MuSK-containing scaffold. Neuron 18, 62–636.
BISBY, M. A. & TETZLAFF, W. (1992) Changes in cytoskeletal protein synthesis following axon injury and during axon regeneration. Molecular Neurobiology 6, 10–123.
BOMZE, H. M., BULSARA, K. R., ISKANDAR, B. J., CARONI, P. & SKENE, J. H. (2001) Spinal axon regeneration evoked by replacing two growth cone proteins in adult neurons. Nature Neuroscience 4, 3–43.
BRANDON, E. P., LIN, W., D'AMOUR, K. A., PIZZO, D. P., DOMINGUEZ, B., SUGIURA, Y., THODE, S., KO, C. P., THAL, L. J., GAGE, F. H. & LEE, K. F. (2003) Aberrant patterning of neuromuscular synapses in choline acetyltransferase-deficient mice. Journal of Neuroscience 23, 53–549.
BROWN, M. C. (1984) Sprouting of motor nerves in adult muscles: A recapitulation of ontogeny. Trends Neuroscience 7, 1–14.
BURGESS, R. W., NGUYEN, Q. T., SON, Y. J., LICHTMAN, J. W. & SANES, J. R. (1999) Alternatively spliced isoforms of nerve-and muscle-derived agrin: Their roles at the neuromuscular junction. Neuron 23, 3–44.
BURKE, R. E. (1994) Physiology of motor units. In Myology (edited by ENGEL, A. G. & FRANZINIARMSTRONG, C.) pp. 46–484. New York: McGraw-Hill.
CARONI, P. & BECKER, M. (1992) The downregulation of growth-associated proteins in motoneurons at the onset of synapse elimination is controlled by muscle activity and IGF1. Journal of Neuroscience 12, 384–3861.
CARONI, P. (1997a) Overexpression of growth-associated proteins in the neurons of adult transgenic mice. Journal of Neuroscience Methods 71, –9.
CARONI, P. (1997b) Intrinsic neuronal determinants that promote axonal sprouting and elongation. Bioessays 19, 76–775.
CARONI, P., AIGNER, L. & SCHNEIDER, C. (1997) Intrinsic neuronal determinants locally regulate extrasynaptic and synaptic growth at the adult neuromuscular junction. Journal of Cell Biology 136, 67–692.
CHIU, A. Y., ZHAI, P., DAL CANTO, M. C., PETERS, T. M., KWON, Y. W., PRATTIS, S. M. & GURNEY, M. E. (1995) Age-dependent penetrance of disease in a transgenic mouse model of familial amyotrophic lateral sclerosis. Molecular Cellular Neuroscience 6, 34–362.
DAVIS, G. W. & GOODMAN, C. S. (1998) Synapse-specific control of synaptic efficacy at the terminals of a single neuron. Nature 392, 8–86.
DAVIS, G.W. (2000) The making of a synapse: Targetderived signals and presynaptic differentiation. Neuron 26, 55–554.
DE PAIVA, A., MEUNIER, F. A., MOLGO, J., AOKI, K. R. & DOLLY, J. O. (1999) Functional repair of motor endplates after botulinum neurotoxin type A poisoning: Biphasic switch of synaptic activity between nerve sprouts and their parent terminals. Precedures of the National Academy of Sciences USA 96, 320–3205.
DEPAOLA, V., ARBER, S. & CARONI, P. (2003) Dynamic equilibrium of presynaptic terminal numbers regulated by AMPA-receptor activation in mature hippocampal networks. Nature Neuroscience 6, 49–500.
FITZSIMONDS, R. M. & POO, M. M. (1998) Retrograde signaling in the development and modification of synapses. Physiology Reviews 78, 14–170.
FREY, D., SCHNEIDER, C., XU, L., BORG, J., SPOOREN, W. & CARONI, P. (2000a) Early and selective loss of neuromuscular synapse subtypes with low sprouting competence in motoneuron diseases. Journal of Neuroscience 20, 253–2542.
FREY, D., LAUX, T., XU, L., SCHNEIDER, C. & CARONI, P. (2000b) Shared and unique roles of CAP23 and GAP43 in actin regulation, neurite outgrowth, and anatomical plasticity. Journal of Cell Biology 149, 144–1454.
FRUGIER, T., NICOLE, S., CIFUENTES-DIAZ, C. & MELKI, J. (2002) The molecular bases of spinal muscular atrophy. Current Opinion in Genetics and Development 12, 29–298.
GAUTAM, M., NOAKES, P. G., MUDD, J., NICHOL, M., CHU, G. C., SANES, J. R. & MERLIE, J. P. (1995) Failure of postsynaptic specialization to develop at neuromuscular junctions of rapsyn-deficient mice. Nature 377, 23–236.
GAUTAM, M., NOAKES, P. G., M OSCOSO, L., RUPP, F., SCHELLER, R. H., MERLIE, J. P. & SANES, J. R. (1996) Defective neuromuscular synaptogenesis in agrindeficient mutant mice. Cell 85, 52–535.
GOLDBERG, J. L., KLASSEN, M. P., HUA, Y. & BARRES, B. A. (2002a) Amacrine-signaled loss of intrinsic axon growth ability by retinal ganglion cells. Science 296, 186–1864.
GOLDBERG, J. L., ESPINOSA, J. S., XU, Y., DAVIDSON, N., KOVACS, G. T. & BARRES, B. A. (2002b) Retinal ganglion cells do not extend axons by default: Promotion by neurotrophic signaling and electrical activity. Neuron 33, 68–702.
GONZALEZ, M., RUGGIERO, F. P., CHANG, Q., SHI, Y. J., RICH, M. M., KRANER, S. & BALICEGORDON, R. J. (1999) Disruption of Trkb-mediated signaling induces disassembly of postsynaptic receptor clustering at neuromuscular junctions. Neuron 24, 56– 583.
GURNEY, M. E., PU, H., CHIU, A. Y., DAL CANTO, M. C., POLCHOW, C. Y., ALEXANDER, D. D., CALIENDO, J., HENTATI, A., KWON, Y. W., DENG, H. X., CHEN, W., ZHAI, P., SUFIT, R. L. & SIDDIQUE, T. (1994) Motor neuron degeneration in mice that express a human Cu, Zn superoxide dismutase mutation. Science 264, 177–1775.
HEATHCOTE, R. D., EKMAN, J. M., CAMPBELL, K. P. & GODFREY, E. W. (2000) Dystroglycan overexpression in vivo alters acetylcholine receptor aggregation at the neuromuscular junction. Developmental Biology 227, 59–605.
HERRERA, A. A., QIANG, H. & KO, C. P. (2000) The role of perisynaptic Schwann cells in development of neuromuscular junctions in the frog (Xenopus laevis). Journal of Neurobiology 45, 23–254.
JACOBSON, C., CÔTÉ, P. D., ROSSI, S. G., ROTUNDO, R. L. & CARBONETTO, S. (2001) The dystroglycan complex is necessary for stabilization of acetylcholine receptor clusters at the neuromuscular junctions and formation of the synaptic basement membrane. Journal of Cell Biology 152, 43–450.
JONES C. L. (1979) The morphogenesis of the thigh of the mouse with special reference to tetrapod muscle homologies. Journal of Morphology 162, 27–309.
LIN, W., BURGESS, R. W., GOMINGUEZ, B., PFAFF, S. L., SANES, J. R. & LEE, K. F. (2001) Distinct roles of nerve and muscle in postsynaptic differentiation of the neuromuscular synapse. Nature 410, 105–1064.
LIVET, J., SIGRIST, M., STROEBEL, S., DEPAOLA., V., PRICE, S. R., HENDERSON, C. E., JESSELL, T. M. & ARBER, S. (2002) ETS gene Pea3 controls the central position and terminal arborization of specific motor neuron pools. Neuron 35, 87–892.
LOVE, F. M. & THOMPSON, W. J. (1999) Glial cells promote muscle reinnervation by responding to activity-dependent postsynaptic signals. J. Neurosci. 19, 1039–10396.
MARANGI, P. A., WIELAND, S. T. & FUHRER, C. (2002) Laminin-1 redistributes postsynaptic proteins and requires rapsyn, tyrosine phosphorylation, and Src and Fyn to stably cluster acetylcholine receptors. Journal of Cell Biology 157, 88–895.
MCKINNEY, R. A., CAPOGNA, M., DURR, R., GAEHWILER, B. H. & THOMPSON, S. M. (1999) Miniature synaptic events maintain dendritic spines via AMPA receptor activation. Nature Neuroscience 2, 4–49.
MISGELD, T., BURGESS, R. W., L EWIS, R. M., CUNNINGHAM, J. M., LICHTMAN, J. W. & SANES, J. R. (2002) Roles of neurotransmitter in synapse formation: Development of neuromuscular junctions lacking choline acetyltransferase. Neuron 36, 63–648.
O'ROURKE, N. A., CLINE, H. T. & FRASER, S. E. (1994) Rapid remodeling of retinal arbors in the tectum with and without blockade of synaptic transmission. Neuron 12, 92–934.
PINTER, M. J., WALDECK, R. F., WALLACE, N. & CORK, L. C. (1995) Motor unit behavior in canine motor neuron disease. Journal of Neuroscience 15, 344–3457.
PINTER, M. J., WALDECK, R. F., COPE, T. C. & CORK, L. C. (1997) Effects of 4-aminopyridine on muscle and motor unit force in canine motor neuron disease. Journal of Neuroscience 17, 450–4507.
PUN, S., SIGRIST, M., SANTOS, A. F., RUEGG, M. A., SANES, J. R., JESSELL, T. M., ARBER, S. & CARONI, P. (2002) An intrinsic distinction in neuromuscular junction assembly and maintenance in different skeletal muscles. Neuron 34, 35–370.
RAFF, M. C., WHITMORE, A. V. & FINN, J. T. (2002) Axonal self-destruction and neurodegeneration. Science 296, 86–871.
REYNOLDS, M. L. & WOOLF, C. J. (1992) Terminal Schwann cells elaborate extensive processes following denervation of the motor endplate. Journal of Neurocytology 21, 5–66.
RICH, M. M., WANG, X., COPE, T. C. & PINTER, M. J. (2002a) Reduced neuromuscular quantal content with normal synaptic release time course and depression in canine motor neuron disease. Journal of Neurophysiology 88, 330–3314.
RICH, M. M., WALDECK, R. F., CORK, L. C., BALICEGORDON, R. J., FYFFE, R. E., WANG, X., COPE, T. C. & PINTER, M. J. (2002b) Reduced endplate currents underlie motor unit dysfunction in canine motor neuron disease. Journal of Neurophysiology 88, 329–32304.
ROBITAILLE, R. (1998) Modulation of synaptic efficacy and synaptic depression by glial cells at the frog neuromuscular junction. Neuron 21, 84–855.
SAGOT, Y., DUBOIS-DAUPHIN, M., TAN, S. A., DE BILBAO, F., AEBISCHER, P., MARTINOU, J. C. & KATO, A. C. (1995) Bcl-2 overexpression prevents motoneuron cell body loss but not axonal degeneration in a mouse model of a neurodegenerative disease. Journal of Neuroscience 15, 772–7733.
SAGOT, Y., TAN, S. A., HAMMANG, J. P., AEBISCHER, P. & KATO, A. C. (1996) GDNF slows loss of motoneurons but not axonal degeneration or premature death ofpmn/pmnmice. Journal of Neuroscience 16, 233– 2341.
SAGOT, Y., ROSSE, T., VEJSADA, R., PERRELET, D. & KATO, A. C. (1998) Differential effects of neurotrophic factors on motoneuron retrograde labeling in a murine model of motoneuron disease. Journal of Neuroscience 18, 113–1141.
SANDROCK, A. W. JR., DRYER, S. E., ROSEN, K. M., GOZANI, S. N., KRAMER, R., THEILL, L. E. & FISCHBACH, G. D. (1997) Maintenance of acetylcholine receptor number by neuregulins at the neuromuscular junction in vivo. Science 276, 59–603.
SANES, J. R. & LICHTMAN, J. W. (1999) Development of the vertebrate neuromuscular junction. Annual Reviews of Neuroscience 22, 38–442.
SANES, J. R. & LICHTMAN, J. W. (2001) Induction, assembly, maturation and maintenance of a postsynaptic apparatus. Nature Reviews Neuroscience 2, 79–805.
SIEGEL, S. G., PATTON, B. & ENGLISH, A. W. (2000) Ciliary neurotrophic factor is required for motoneuron sprouting. Experimental Neurology 166, 20–212.
SKENE, J. H. P. (1989) Axonal growth-associated proteins. Annual Reviews Neuroscience 12, 12–156.
SKORPEN, J., LAFOND-BENESTAD, S. & LOMO, T. (1999) Regulation of the size and distribution of ectopic neuromuscular junctions in adult skeletal muscle by nerve-derived trophic factor and electrical muscle activity. Molecular Cellular Neuroscience 13, 19–206.
SON, Y. J. & THOMPSON, W. J. (1995) Nerve sprouting in muscle is induced and guided by processes extended by Schwann cells. Neuron 14, 13–141.
TAM, S. L., ARCHIBALD, V., TYREMAN, N. & GORDON, T. (2001) Increased neuromuscular activity reduces sprouting in partially denervated muscles. Journal of Neuroscience 21, 65–667.
VERHAGE, M., MAIA, A. S., PLOMP, J. J., BRUSSARD, A. B., HEEROMA, J. H., VERMEER, H., TOONEN, R. F., HAMMER, R. E., VAN DEN BERG, T. K., MISSLER, M. et al. (2000) Synaptic assembly of the brain in the absence of neurotransmitter secretion. Science 287, 86–869.
WESTON, C., YEE, B, HOD, E. & PRIVES, J. (2000) Agrininduced acetylcholine receptor clustering is mediated by the small guanosine triphosphatases Rac and Cdc42. Journal of Cell Biology 150, 20–212.
WIDMER, F. & CARONI, P. (1990) Identification, localization, and primary structure of CAP-23, a particle-bound cytosolic protein of early development. Journal of Cell Biology 111, 303–3047.
WOLDEYESUS, M. T., BRITSCH, S., RIETHMACHER, D., XU, L., SONNENBERG-RIETHMACHER, E., ABOU-REBYEH, F., HARVEY, R., CARONI, P. & BIRCHMEIER, C. (1999) Peripheral nervous system defects in erbB2 mutants following genetic rescue of heart development. Genes and Development 13, 253– 2548.
YANG, J. F., CAO, G., KOIRALA, S., REDDY, L. V. & KO, C. P. (2001a) Schwann cells express active agrin and enhance aggregation of acetylcholine receptors on muscle fibers. Journal of Neuroscience 21, 957–9584.
YANG, X., ARBER, S., WILLIAM, C., LI, L., TANABE, Y., JESSELL, T. M., BIRCHMEIER, C. & BURDEN, S. J. (2001b) Patterning of muscle acetylcholine receptor gene expression in the absence of motor innervation. Neuron 30, 39–410.
ZHEN, G. L., WANG, Q., ZHOU, J. Z., WANG, J., LUO, Z., LIU, M., HE, X., WYNSCHAW-BORIS, A., XIONG, W. C., LU, B. & MEI, L. (2002) Regulation of AChR clustering by dishevelled interacting with MuSK and PAK1. Neuron 35, 48–505.
ZITO, K. & SVOBODA, K. (2002) Activity-dependent synaptogenesis in the adult Mammalian cortex. Neuron 35, 101–1017.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Santos, A.F., Caroni, P. Assembly, plasticity and selective vulnerability to disease of mouse neuromuscular junctions. J Neurocytol 32, 849–862 (2003). https://doi.org/10.1023/B:NEUR.0000020628.36013.88
Issue Date:
DOI: https://doi.org/10.1023/B:NEUR.0000020628.36013.88