Abstract
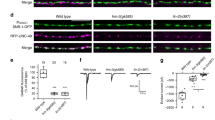
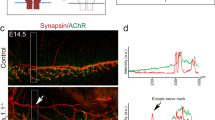
The receptor tyrosine kinase MuSK is activated by agrin, an extracellular matrix protein believed to be utilized by motoneurons to regulate the formation or maintenance of the neuromuscular junction (NMJ). Recent studies have shed light on intracellular signaling mechanisms downstream of MuSK. Agrin enhances the activity of Rho GTPases and PAK, which is required for AChR clustering. Activation of these enzymes requires not only the kinase activity of MuSK, but also its interaction with proteins such as Dishevelled. These results suggest that MuSK may function as a scaffold tyrosine kinase that forms a multi-molecule complex for AChR clustering.
Similar content being viewed by others
References
ANDERSON, M. J. & COHEN, M. W. (1977) Nerveinduced and spontaneous redistribution of acetylcholine receptors on cultured muscle cells. J Physiol 268, 75–773.
APEL, E. D., LEWIS, R. M., GRADY, R. M. & SANES, J. R. (2000) Syne-1, a dystrophin-and Klarsicht-related protein associated with synaptic nuclei at the neuromuscular junction. Journal of Biological Chemistry 275, 3198–31995.
APEL, E. D., ROBERDS, S. L., CAMPBELL, K. P. & MERLIE, J. P. (1995) Rapsyn may function as a link between the acetylcholine receptor and the agrin-binding dystrophin-associated glycoprotein complex. Neuron 15, 11–126.
BIXBY, J. L., BAERWALD-DE LA TORRE, K., WANG, C., RATHJEN, F. G. & RUEGG, M. A. (2002) A neuronal inhibitory domain in the N-terminal half of agrin. Journal of Neurobiology 50, 16–179.
BLOCH, R. J. (1986) Actin at receptor-rich domains of isolated acetylcholine receptor clusters. Journal of Cell Biology 102, 144–1458.
BLOCH, R. J., BEZAKOVA, G., URSITTI, J. A., ZHOU, D. & PUMPLIN, D. W. (1997) A membrane skeleton that clusters nicotinic acetylcholine receptors in muscle. Society of General Physiologists Series 52, 17–195.
BORGES, L. S. & FERNS, M. (2001) Agrin-induced phosphorylation of the acetylcholine receptor regulates cytoskeletal anchoring and clustering. Journal of Cell Biology 153, –12.
BOSE, C. M., QIU, D., BERGAMASCHI, A., GRAVANTE, B., BOSSI, M., VILLA, A., RUPP, F. & MALGAROLI, A. (2000) Agrin controls synaptic differentiation in hippocampal neurons. Journal of Neuroscience 20, 908–9095.
BOWE, M. A., DEYST, K. A., LESZYK, J. D. & FALLON, J. R. (1994) Identification and purification of an agrin receptor from Torpedo postsynaptic membranes: A heteromeric complex related to the dystroglycans. Neuron 12, 117–1180.
BOWEN, D. C., SUGIYAMA, J., FERNS, M. & HALL, Z. W. (1996) Neural agrin activates a high-affinity receptor in C2 muscle cells that is unresponsive to muscle agrin. Journal of Neuroscience 16, 379–3797.
BURDEN, S. J. (2000) Wnts as retrograde signals for axon and growth cone differentiation. Cell 100, 49–497.
BURDEN, S. J. (2002) Building the vertebrate neuromuscular synapse. Journal of Neurobiology 53, 50–511.
BURGESS, R. W., SKARNES, W. C. & SANES, J. R. (2000) Agrin isoforms with distinct amino termini: Differential expression, localization, and function. Journal of Cell Biology 151, 4–52.
BURKIN, D. J., GU, M., HODGES, B. L., CAMPANELLI, J. T. & KAUFMAN, S. J. (1998) A functional role for specific spliced variants of the alpha7beta1 integrin in acetylcholine receptor clustering. Journal of Cell Biology 143, 106–1075.
CADIGAN, K. M. & NUSSE, R. (1997) Wnt signaling: A common theme in animal development. Genes Dev 11, 328–3305.
CAMPAGNA, J. A., RUEGG, M. A. & BIXBY, J. L. (1995) Agrin is a differentiation-inducing “stop signal” for motoneurons in vitro. Neuron 15, 136–1374.
CAMPANELLI, J. T., GAYER, G. G. & SCHELLER, R. H. (1996) Alternative RNA splicing that determines agrin activity regulates binding to heparin and alphadystroglycan. Development 122, 166–1672.
CAMPANELLI, J. T., HOCH, W., RUPP, F., KREINER, T. & SCHELLER, R. H. (1991) Agrin mediates cell contact-induced acetylcholine receptor clustering. Cell 67, 90–916.
CAMPANELLI, J. T., ROBERDS, S. L., CAMPBELL, K. P. & SCHELLER, R. H. (1994) A role for dystrophin-associated glycoproteins and utrophin in agrin-induced AChR clustering. Cell 77, 66–674.
CARLIER, M. F., RESSAD, F. & PANTALONI, D. (1999) Control of actin dynamics in cell motility. Role of ADF/cofilin. Journal of Biological Chemistry 274, 3382–33830.
CAU, J., FAURE, S., COMPS, M., DELSERT, C. & MORIN, N. (2001) A novel p21-activated kinase binds the actin and microtubule networks and induces microtubule stabilization. Journal of Cell Biology 155, 102– 1042.
CHANG, D., WOO, J. S., CAMPANELLI, J., SCHELLER, R. H. & IGNATIUS, M. J. (1997) Agrin inhibits neurite outgrowth but promotes attachment of embryonic motor and sensory neurons. Developmental Biology 181, 2–35.
COHEN, I., RIMER, M., LOMO, T. & MCMAHAN, U. J. (1997a) Agrin-induced postsynaptic-like apparatus in skeletal muscle fibers in vivo. Molecular and Cellular Neurosciences 9, 23–253.
COHEN, M. W., JACOBSON, C., YURCHENCO, P. D., MORRIS, G. E. & CARBONETTO, S. (1997b) Laminin-induced clustering of dystroglycan on embryonic muscle cells: Comparison with agrin-induced clustering. Journal of Cell Biology 136, 104–1058.
COOPER, J. A. & SCHAFER, D. A. (2000) Control of actin assembly and disassembly at filament ends. Current Opinion in Cell Biology 12, 9–103.
COTMAN, S. L., HALFTER, W. & COLE, G. J. (1999) Identification of extracellular matrix ligands for the heparan sulfate proteoglycan agrin. Experimental Cell Research 249, 5–64.
COTMAN, S. L., HALFTER, W. & COLE, G. J. (2000) Agrin binds to beta-amyloid (Abeta), accelerates abeta fibril formation, and is localized to Abeta deposits in Alzheimer's disease brain. Molecular and Cellular Neurosciences 15, 18–198.
COUTEAUX, R. (1973) Motor endplate structure. In Structure and Function of Muscle (edited by H., B. G.). New York: Academic Press.
DAGGETT, D. F., COHEN, M. W., STONE, D., NIKOLICS, K., RAUVALA, H. & PENG, H. B. (1996) The role of an agrin-growth factor interaction in ACh receptor clustering. Molecular and Cellular Neurosciences 8, 27–285.
DAI, Z., LUO, X., XIE, H. & PENG, H. B. (2000) The actindriven movement and formation of acetylcholine receptor clusters. Journal of Cell Biology 150, 132–1334.
DANN, C. E., HSIEH, J. C., RATTNER, A., SHARMA, D., NATHANS, J. & LEAHY, D. J. (2001) Insights into Wnt binding and signalling from the structures of two Frizzled cysteine-rich domains. Nature 412, 8–90.
DAVIS, G. W. (2000) The making of a synapse: Target-derived signals and presynaptic differentiation. Neuron 26, 55–554.
DECHIARA, T. M., BOWEN, D. C., VALENZUELA, D. M., SIMMONS, M. V., POUEYMIROU, W. T., THOMAS, S., KINETZ, E., COMPTON, D. L., ROJAS, E., PARK, J. S., SMITH, C., DISTEFANO, P. S., GLASS, D. J., BURDEN, S. J. & YANCOPOULOS, G. D. (1996) The receptor tyrosine kinase MuSK is required for neuromuscular junction formation in vivo. Cell 85, 50–512.
DENZER, A. J., SCHULTHESS, T., FAUSER, C., SCHUMACHER, B., KAMMERER, R. A., ENGEL, J. & RUEGG, M. A. (1998) Electron microscopic structure of agrin and mapping of its binding site in laminin-1. EMBO Journal 17, 33–343.
DRACHMAN, D. B. (1994) Myasthenia gravis. New England Journal of Medicine 330, 179–1810.
EDWARDS, D. C., SANDERS, L. C., BOKOCH, G. M. & GILL, G. N. (1999) Activation of LIM-kinase by Pak1 couples Rac/Cdc42 GTPase signalling to actin cytoskeletal dynamics. Nature Cell biology 1, 25–259.
ENGEL, A. G., OHNO, K. & SINE, S. M. (2003) Congenital myasthenic syndromes: Progress over the past decade. Muscle Nerve 27, –25.
FENG, G., LASKOWSKI, M. B., FELDHEIM, D. A., WANG, H., LEWIS, R., FRISEN, J., FLANAGAN, J. G. & SANES, J. R. (2000) Roles for ephrins in positionally selective synaptogenesis between motor neurons and muscle fibers. Neuron 25, 29–306.
FERNS, M., DEINER, M. & HALL, Z. (1996) Agrin-induced acetylcholine receptor clustering in mammalian muscle requires tyrosine phosphorylation. Journal of Cell Biology 132, 93–944.
FERNS, M., HOCH, W., CAMPANELLI, J. T., RUPP, F., HALL, Z. W. & SCHELLER, R. H. (1992) RNA splicing regulates agrin-mediated acetylcholine receptor clustering activity on cultured myotubes. Neuron 8, 107–1086.
FERNS, M. J., CAMPANELLI, J. T., HOCH, W., SCHELLER, R. H. & HALL, Z. (1993) The ability of agrin to cluster AChRs depends on alternative splicing and on cell surface proteoglycans. Neuron 11, 49–502.
FERREIRA, A. (1999) Abnormal synapse formation in agrindepleted hippocampal neurons. Journal of Cell Science 112 (Pt 24), 472–4738.
FRAIL, D. E., MCLAUGHLIN, L. L., MUDD, J. & MERLIE, J. P. (1988) Identification of the mouse muscle 43,000-dalton acetylcholine receptor-associated protein (RAPsyn) by cDNA cloning. Journal of Biological Chemistry 263, 1560–15607.
FRANK, E. & FISCHBACH, G. D. (1979) Early events in neuromuscular junction formation in vitro: Induction of acetylcholine receptor clusters in the postsynaptic membrane and morphology of newly formed synapses. Journal of Cell Biology 83, 14–158.
FROEHNER, S. C., LUETJE, C. W., SCOTLAND, P. B. & PATRICK, J. (1990) The postsynaptic 43K protein clusters muscle nicotinic acetylcholine receptors in Xenopus oocytes. Neuron 5, 40–410.
FU, A. K., SMITH, F. D., ZHOU, H., CHU, A. H., TSIM, K. W., PENG, B. H. & IP, N. Y. (1999) Xenopus musclespecific kinase: Molecular cloning and prominent expression in neural tissues during early embryonic development. European Journal of Neuroscience 11, 37–382.
FUHRER, C. & HALL, Z. W. (1996) Functional interaction of Src family kinases with the acetylcholine receptor in C2 myotubes. Journal of Biological Chemistry 271, 3247–32481.
FUHRER, C., SUGIYAMA, J. E., TAYLOR, R. G. & HALL, Z. W. (1997) Association of muscle-specific kinaseMuSK with the acetylcholine receptor in mammalian muscle. EMBO Journal 16, 495–4960.
GANJU, P., WALLS, E., BRENNAN, J. & REITH, A. D. (1995) Cloning and developmental expression of Nsk2, a novel receptor tyrosine kinase implicated in skeletal myogenesis. Oncogene 11, 28–290.
GAUTAM, M., NOAKES, P. G., MOSCOSO, L., RUPP, F., SCHELLER, R. H., MERLIE, J. P. & SANES, J. R. (1996) Defective neuromuscular synaptogenesis in agrin-deficient mutant mice. Cell 85, 52–535.
GESEMANN, M., DENZER, A. J. & RUEGG, M. A. (1995) Acetylcholine receptor-aggregating activity of agrin isoforms and mapping of the active site. Journal of Cell Biology 128, 62–636.
GINGRAS, J., RASSADI, S., COOPER, E. & FERNS, M. (2002) Agrin plays an organizing role in the formation of sympathetic synapses. Journal of Cell Biology 158, 110–1118.
GLASS, D. J., APEL, E. D., SHAH, S., BOWEN, D. C., DECHIARA, T. M., STITT, T. N., SANES, J. R. & YANCOPOULOS, G. D. (1997) Kinase domain of the muscle-specific receptor tyrosine kinase (MuSK) is sufficient for phosphorylation but not clustering of acetylcholine receptors: Required role for the MuSK ectodomain. Proceedings of the National Academy of Sciences of the United States of America 94, 884–8853.
GLASS, D. J., BOWEN, D. C., STITT, T. N., RADZIEJEWSKI, C., BRUNO, J., RYAN, T. E., GIES, D. R., SHAH, S., MATTSSON, K., BURDEN, S. J., DISTEFANO, P. S., VALENZUELA, D. M., DECHIARA, T. M. & YANCOPOULOS, G. D. (1996) Agrin acts via a MuSK receptor complex. Cell 85, 51–523.
GODFREY, E. W., NITKIN, R. M., WALLACE, B. G., RUBIN, L. L. & MCMAHAN, U. J. (1984) Components of Torpedo electric organ and muscle that cause aggregation of acetylcholine receptors on cultured muscle cells. Journal of Cell Biology 99, 61–627.
HABAS, R., KATO, Y. & HE, X. (2001) Wnt/Frizzled activation of Rho regulates vertebrate gastrulation and requires a novel Formin homology protein Daam1. Cell 107, 84–854.
HALFTER, W., SCHURER, B., YIP, J., YIP, L., TSEN, G., LEE, J. A. & COLE, G. J. (1997) Distribution and substrate properties of agrin, a heparan sulfate proteoglycan of developing axonal pathways. Journal of Comparative Neurology 383, –17.
HALL, A. C., LUCAS, F. R. & SALINAS, P. C. (2000) Axonal remodeling and synaptic differentiation in the cerebellum is regulated by WNT-7a signaling. Cell 100, 52–535.
HERBST, R., AVETISOVA, E. & BURDEN, S. J. (2002) Restoration of synapse formation in Musk mutant mice expressing a Musk/Trk chimeric receptor. Development 129, 544–5460.
HERBST, R. & BURDEN, S. J. (2000) The juxtamembrane region of MuSK has a critical role in agrin-mediated signaling. EMBO Journal 19, 6–77.
HOCH, W., CAMPANELLI, J. T. & SCHELLER, R. H. (1994) Agrin-induced clustering of acetylcholine receptors: A cytoskeletal link. Journal of Cell Biology 126, –4.
HOCH, W., FERNS, M., CAMPANELLI, J. T., HALL, Z. W. & SCHELLER, R. H. (1993) Developmental regulation of highly active alternatively spliced forms of agrin. Neuron 11, 47–490.
HOPF, C. & HOCH, W. (1996) Agrin binding to alpha-dystroglycan. Domains of agrin necessary to induce acetylcholine receptor clustering are overlapping but not identical to the alpha-dystroglycan-binding region. Journal of Biological Chemistry 271, 523–5236.
IP, F. C., GLASS, D. G., GIES, D. R., CHEUNG, J., LAI, K. O., FU, A. K., YANCOPOULOS, G. D. & IP, N. Y. (2000) Cloning and characterization of muscle-specific kinase in chicken. Molecular and Cellular Neurosciences 16, 66–673.
JENNINGS, C. G., DYER, S. M. & BURDEN, S. J. (1993) Muscle-specific trk-related receptor with a kringle domain defines a distinct class of receptor tyrosine kinases. Proceedings of the National Academy of Sciences of the United States of America 90, 289–2899.
JONES, G., MEIER, T., LICHTSTEINER, M., WITZEMANN, V., SAKMANN, B. & BRENNER, H. R. (1997) Induction by agrin of ectopic and functional postsynaptic-like membrane in innervated muscle. Proceedings of the National Academy of Sciences of the United States of America 94, 265–2659.
JONES, M. A. & WERLE, M. J. (2000) Nitric oxide is a downstream mediator of agrin-induced acetylcholine receptor aggregation. Molecular and Cellular Neurosciences 16, 64–660.
KAMMERER, R. A., SCHULTHESS, T., LANDWEHR, R., SCHUMACHER, B., LUSTIG, A., YURCHENCO, P. D., RUEGG, M. A., ENGEL, J. & DENZER, A. J. (1999) Interaction of agrin with laminin requires a coiled-coil conformation of the agrin-binding site within the laminin gamma1 chain. EMBO Journal 18, 676–6770.
KLEIN, P. S. & MELTON, D. A. (1996) A molecular mechanism for the effect of lithium on development. Proceedings of the National Academy of Sciences of the United States of America 93, 845–8459.
KLINGENSMITH, J. & NUSSE, R. (1994) Signaling by wingless in Drosophila. Developmental Biology 166, 39–414.
KORENBAUM, E. & RIVERO, F. (2002) Calponin homology domains at a glance. Journal of Cell Science 115, 354–3545.
LIN, W., BURGESS, R. W., DOMINGUEZ, B., PFAFF, S. L., SANES, J. R. & LEE, K. F. (2001) Distinct roles of nerve and muscle in postsynaptic differentiation of the neuromuscular synapse. Nature 410, 105–1064.
LUBIT, B. W. (1984) Association of beta-cytoplasmic actin with high concentrations of acetylcholine receptor (AChR) in normal and anti-AChR-treated primary rat muscle cultures. Journal of Histochemistry and Cytochemistry 32, 97–981.
LUCK, G., HOCH, W., HOPF, C. & BLOTTNER, D. (2000) Nitric oxide synthase (NOS-1) coclustered with agrin-induced AChR-specializations on cultured skeletal myotubes. Molecular and Cellular Neurosciences 16, 26–281.
LUO, Z. G., WANG, Q., ZHOU, J. Z., WANG, J., LUO, Z., LIU, M., HE, X., WYNSHAW-BORIS, A., XIONG, W. C., LU, B. & MEI, L. (2002) Regulation ofAChRclustering by Dishevelled interacting with MuSK and PAK1. Neuron 35, 48–505.
LUPA, M. T. & HALL, Z. W. (1989) Progressive restriction of synaptic vesicle protein to the nerve terminal during development of the neuromuscular junction. Journal of Neuroscience 9, 393–3945.
MA, E., MORGAN, R. & GODFREY, E. W. (1994) Distribution of agrin mRNAs in the chick embryo nervous system. Journal of Neuroscience 14, 294–2952.
MA, E., MORGAN, R. & GODFREY, E. W. (1995) Agrin mRNA variants are differentially regulated in developing chick embryo spinal cord and sensory ganglia. Journal of Neurobiology 26, 58–597.
MANTYCH, K. B. & FERREIRA, A. (2001) Agrin differentially regulates the rates of axonal and dendritic elongation in cultured hippocampal neurons. Journal of Neuroscience 21, 680–6809.
MARTIN, P. T. & SANES, J. R. (1997) Integrins mediate adhesion to agrin and modulate agrin signaling. Development 124, 390–3917.
MARTIN, P. T., SCOTT, L. J., PORTER, B. E. & SANES, J. R. (1999) Distinct structures and functions of related pre-and postsynaptic carbohydrates at the mammalian neuromuscular junction. Molecular and Cellular Neurosciences 13, 10–118.
MASIAKOWSKI, P. & YANCOPOULOS, G. D. (1998) The Wnt receptor CRD domain is also found in MuSK and related orphan receptor tyrosine kinases. Current Biology 8, R407.
MCMAHAN, U. J. (1990) The agrin hypothesis. Cold Spring Harbor Symposia on Quantitative Biology 55, 40–418.
MCMAHAN, U. J., HORTON, S. E., WERLE, M. J., HONIG, L. S., KROGER, S., RUEGG, M. A. & ESCHER, G. (1992) Agrin isoforms and their role in synaptogenesis. Current Opinion in Cell Biology 4, 86–874.
MEYER, G. & WALLACE, B. G. (1998) Recruitment of a nicotinic acetylcholine receptor mutant lacking cytoplasmic tyrosine residues in its beta subunit into agrin-induced aggregates. Molecular and Cellular Neurosciences 11, 32–333.
MITTAUD, P., MARANGI, P. A., ERB-VOGTLI, S. & FUHRER, C. (2001) Agrin-induced activation of acetylcholine receptor-bound Src family kinases requires Rapsyn and correlates with acetylcholine receptor clustering. Journal of Biological Chemistry 276, 1450–14513.
MOHAMED, A. S., RIVAS-PLATA, K. A., KRAAS, J. R., SALEH, S. M. & SWOPE, S. L. (2001) Src-class kinases act within the agrin/MuSK pathway to regulate acetylcholine receptor phosphorylation, cytoskeletal anchoring, and clustering. Journal of Neuroscience 21, 380– 3818.
MOHAMED, A. S. & SWOPE, S. L. (1999) Phosphorylation and cytoskeletal anchoring of the acetylcholine receptor by Src class protein-tyrosine kinases. Activation by rapsyn. Journal of Biological Chemistry 274, 2052–20539.
MONTANARO, F., GEE, S. H., JACOBSON, C., LINDENBAUM, M. H., FROEHNER, S. C. & CARBONETTO, S. (1998) Laminin and alpha-dystroglycan mediate acetylcholine receptor aggregation via a MuSK-independent pathway. Journal of Neuroscience 18, 125–1260.
MORRIS, J. K., LIN, W., HAUSER, C., MARCHUK, Y., GETMAN, D. & LEE, K. F. (1999) Rescue of the cardiac defect in ErbB2 mutant mice reveals essential roles of ErbB2 in peripheral nervous system development. Neuron 23, 27–283.
NITKIN, R. M., SMITH, M. A., MAGILL, C., FALLON, J. R., YAO, Y. M., WALLACE, B. G. & MCMAHAN, U. J. (1987) Identification of agrin, a synaptic organizing protein fromTorpedo electric organ. Journal of Cell Biology 105, 247–2478.
NOAKES, P. G., GAUTAM, M., MUDD, J., SANES, J. R. & MERLIE, J. P. (1995) Aberrant differentiation of neuromuscular junctions in mice lacking s-laminin/laminin beta 2. Nature 374, 25–262.
NOORDERMEER, J., KLINGENSMITH, J., PERRIMON, N. & NUSSE, R. (1994) dishevelled and armadillo act in the wingless signalling pathway in Drosophila. Nature 367, 8–83.
O'CONNOR, L. T., LAUTERBORN, J. C., GALL, C. M. & SMITH, M. A. (1994) Localization and alternative splicing of agrin mRNA in adult rat brain: Transcripts encoding isoforms that aggregate acetylcholine receptors are not restricted to cholinergic regions. Journal of Neuroscience 14, 114–1152.
OHNO, K., ENGEL, A. G., SHEN, X. M., SELCEN, D., BRENGMAN, J., HARPER, C. M., TSUJINO, A. & MILONE, M. (2002) Rapsyn mutations in humans cause endplate acetylcholine-receptor deficiency and myasthenic syndrome. American Journal of Human Genetics 70, 87–885.
O'TOOLE, J. J., DEYST, K. A., BOWE, M. A., NASTUK, M. A., MCKECHNIE, B. A. & FALLON, J. R. (1996) Alternative splicing of agrin regulates its binding to heparin alpha-dystroglycan, and the cell surface. Proceedings of the National Academy of Sciences of the United States of America 93, 736–7374.
PACKARD, M., KOO, E. S., GORCZYCA, M., SHARPE, J., CUMBERLEDGE, S. & BUDNIK, V. (2002) The Drosophila Wnt, wingless, provides an essential signal for pre-and postsynaptic differentiation. Cell 111, 31–330.
PENG, H. B., ALI, A. A., DAI, Z., DAGGETT, D. F., RAULO, E. & RAUVALA, H. (1995) The role of heparin-binding growth-associated molecule (HB-GAM) in the postsynaptic induction in cultured muscle cells. Journal of Neuroscience 15, 302–3038.
PENG, H. B., BAKER, L. P. & CHEN, Q. (1991) Induction of synaptic development in cultured muscle cells by basic fibroblast growth factor. Neuron 6, 23–246.
PERRIMON, N. & MAHOWALD, A. P. (1987) Multiple functions of segment polarity genes in Drosophila. Developmental Biology 119, 58–600.
PHILLIPS, W. D. (1995) Acetylcholine receptors and the cytoskeletal connection. Clinical and Experimental Pharmacology and Physiology 22, 96–965.
QU, Z. & HUGANIR, R. L. (1994) Comparison of innervation and agrin-induced tyrosine phosphorylation of the nicotinic acetylcholine receptor. Journal of Neuroscience 14, 683–6841.
RAUVALA, H. & PENG, H. B. (1997) HB-GAM (heparinbinding growth-associated molecule) and heparin-type glycans in the development and plasticity of neuron-target contacts. Progress in Neurobiology 52, 12–144.
RIMER, M., MATHIESEN, I., LOMO, T. & MCMAHAN, U. J. (1997) gamma-AChR/epsilon-AChR switch at agrin-induced postsynaptic-like apparatus in skeletal muscle. Molecular and Cellular Neurosciences 9, 25–263.
RUEGG, M. A., TSIM, K. W., HORTON, S. E., KROGER, S., ESCHER, G., GENSCH, E. M. & MCMAHAN, U. J. (1992) The agrin gene codes for a family of basal lamina proteins that differ in function and distribution. Neuron 8, 69–699.
SALDANHA, J., SINGH, J. & MAHADEVAN, D. (1998) Identification of a Frizzled-like cysteine rich domain in the extracellular region of developmental receptor tyrosine kinases. Protein Science 7, 163–1635.
SANDER, A., HESSER, B. A. & WITZEMANN, V. (2001) MuSK induces in vivo acetylcholine receptor clusters in a ligand-independent manner. Journal of Cell Biology 155, 128–1296.
SANES, J. R. (1997) Genetic analysis of postsynaptic differentiation at the vertebrate neuromuscular junction. Current Opinion in Neurobiology 7, 9–100.
SANES, J. R. & LICHTMAN, J. W. (2001) Induction, assembly, maturation and maintenance of a postsynaptic apparatus. Nature Review Neuroscience 2, 79–805.
SERPINSKAYA, A. S., FENG, G., SANES, J. R. & CRAIG, A. M. (1999) Synapse formation by hippocampal neurons from agrin-deficient mice. Developmental Biology 205, 6–78.
SHARMA, S. K. & WALLACE, B. G. (2003) Lithium inhibits a late step in agrin-induced AChR aggregation. Journal of Neurobiology 54, 34–357.
SMITH, C. L., MITTAUD, P., PRESCOTT, E. D., FUHRER, C. & BURDEN, S. J. (2001) Src, Fyn, and Yes are not required for neuromuscular synapse formation but are necessary for stabilization of agrin-induced clusters of acetylcholine receptors. Journal of Neuroscience 21, 315–3160.
SON, Y. J., TRACHTENBERG, J. T. & THOMPSON, W. J. (1996) Schwann cells induce and guide sprouting and reinnervation of neuromuscular junctions. Trends Neurosci 19, 28–285.
STAMBOLIC, V., RUEL, L. & WOODGETT, J. R. (1996) Lithium inhibits glycogen synthase kinase-3 activity and mimics wingless signalling in intact cells. Current Biology 6, 166–1668.
STARR, D. A. & HAN, M. (2003) ANChors away: An actin based mechanism of nuclear positioning. Journal of Cell Science 116, 21–216.
STONE, D. M. & NIKOLICS, K. (1995) Tissue-and age-specific expression patterns of alternatively spliced agrin mRNA transcripts in embryonic rat suggest novel developmental roles. Journal of Neuroscience 15, 676–6778.
STORMS, S. D., KIM, A. C., TRAN, B. H., COLE, G. J. & MURRAY, B. A. (1996) NCAM-mediated adhesion of transfected cells to agrin. Cell Adhesion and Communication 3, 49–509.
STROCHLIC, L., CARTAUD, A., LABAS, V., HOCH, W., ROSSIER, J. & CARTAUD, J. (2001) MAGI-1c: A synaptic MAGUK interacting with muSK at the vertebrate neuromuscular junction. Journal of Cell Biology 153, 112–1132.
SUGIYAMA, J., BOWEN, D. C. & HALL, Z. W. (1994) Dystroglycan binds nerve and muscle agrin. Neuron 13, 10–115.
SUGIYAMA, J. E., GLASS, D. J., YANCOPOULOS, G. D. & HALL, Z. W. (1997) Laminin-induced acetylcholine receptor clustering: An alternative pathway. Journal of Cell Biology 139, 18–191.
SWOPE, S. L., QU, Z. & HUGANIR, R. L. (1995) Phosphorylation of the nicotinic acetylcholine receptor by protein tyrosine kinases. Annals of the New York Academy of Sciences 757, 19–214.
THEISEN, H., PURCELL, J., BENNETT, M., KANSAGARA, D., SYED, A. & MARSH, J. L. (1994) dishevelled is required during wingless signaling to establish both cell polarity and cell identity. Development 120, 34–360.
THOMPSON, W. J. (2001) Seeing is believing: GFP transgenics illuminate synapse elimination. Neuron 31, 34–342.
VALENZUELA, D. M., STITT, T. N., DISTEFANO, P. S., ROJAS, E., MATTSSON, K., COMPTON, D. L., NUNEZ, L., PARK, J. S., STARK, J. L., GIES, D. R., et al. (1995) Receptor tyrosine kinase specific for the skeletal muscle lineage: Expression in embryonic muscle, at the neuromuscular junction, and after injury. Neuron 15, 57–584.
WALKER, J. H., BOUSTEAD, C. M., WITZEMANN, V., SHAW, G., WEBER, K. & OSBORN, M. (1985) Cytoskeletal proteins at the cholinergic synapse: Distribution of desmin, actin, fodrin, neurofilaments, and tubulin in Torpedo electric organ. European Journal of Cell Biology 38, 12–133.
WALLACE, B. G. (1994) Staurosporine inhibits agrin-induced acetylcholine receptor phosphorylation and aggregation. Journal of Cell Biology 125, 66–668.
WALLACE, B. G. (1995) Regulation of the interaction of nicotinic acetylcholine receptors with the cytoskeleton by agrin-activated protein tyrosine kinase. Journal of Cell Biology 128, 112–1129.
WALLACE, B. G., QU, Z. & HUGANIR, R. L. (1991) Agrin induces phosphorylation of the nicotinic acetylcholine receptor. Neuron 6, 86–878.
WATTY, A., NEUBAUER, G., DREGER, M., ZIMMER, M., WILM, M. & BURDEN, S. J. (2000) The in vitro and in vivo phosphotyrosine map of activated MuSK. Proceedings of the National Academy of Sciences of the United States of America 97, 458–4590.
WESTON, C., GORDON, C., TERESSA, G., HOD, E., REN, X. D. & PRIVES, J. (2003) Cooperative Regulation by Rac and Rhoof Agrin-induced Acetylcholine Receptor Clustering in Muscle Cells. Journal of Biological Chemistry 278, 645–6455.
WESTON, C., YEE, B., HOD, E. & PRIVES, J. (2000) Agrin-induced acetylcholine receptor clustering is mediated by the small guanosine triphosphatases Rac and Cdc42. Journal of Cell Biology 150, 20–212.
WILLMANN, R. & FUHRER, C. (2002) Neuromuscular synaptogenesis: Clustering of acetylcholine receptors revisited. Cellular and Molecular Life Sciences 59, 129– 1316.
XIA, J., ZHANG, X., STAUDINGER, J. & HUGANIR, R. L. (1999) Clustering of AMPA receptors by the synaptic PDZ domain-containing protein PICK1. Neuron 22, 17–187.
XU, Y. K. & NUSSE, R. (1998) The Frizzled CRD domain is conserved in diverse proteins including several receptor tyrosine kinases. Current Biology 8, R40–406.
YANG, J. F., CAO, G., KOIRALA, S., REDDY, L. V. & KO, C. P. (2001a) Schwann cells express active agrin and enhance aggregation of acetylcholine receptors on muscle fibers. Journal of Neuroscience 21, 957–9584.
YANG, X., ARBER, S., WILLIAM, C., LI, L., TANABE, Y., JESSELL, T. M., BIRCHMEIER, C. & BURDEN, S. J. (2001b) Patterning of muscle acetylcholine receptor gene expression in the absence of motor innervation. Neuron 30, 39–410.
YANG, G. C., XIONG, Z. H., LU, W. C. & ME, B. L. (2003) Implication of geranylgeranyl transferase I in syhapse formation. Neuron 40, 40–417.
ZHANG, L., WANG, J. M., TSENG, C. N., VIROONCHATAPAN, N., ROTHE, E., YAO, Y., WANG, Z. Z. (2002) Agrin induces postsynaptic differentiation at the neuromuscular junction by antagonizing the wnt/beta-catenin pathway. Society for Neuroscience Abstract. Program No. 234.12.
ZHOU, H., GLASS, D. J., YANCOPOULOS, G. D. & SANES, J. R. (1999) Distinct domains of MuSK mediate its abilities to induce and to associate with postsynaptic specializations. Journal of Cell Biology 146, 113–1146.
ZHOU, H., MURAMATSU, T., HALFTER, W., TSIM, K. W. K. & PENG, H. B. (1997) A Role of Midkine in the Development of the Neuromuscular Junction. Molecular and Cellular Neurosciences 10, 5–70
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Luo, Z., Wang, Q., Dobbins, G.C. et al. Signaling complexes for postsynaptic differentiation. J Neurocytol 32, 697–708 (2003). https://doi.org/10.1023/B:NEUR.0000020618.65271.63
Issue Date:
DOI: https://doi.org/10.1023/B:NEUR.0000020618.65271.63