Abstract
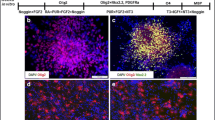
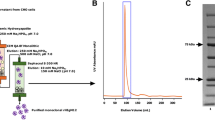
We showed previously that spinal cord implants of hybridoma cells (O1) that secrete an IgM antigalactocerebroside cause focal multiple-sclerosis-like plaques of demyelination followed by remyelination to form “shadow plaques” (Rosenbluth et al., 1999). The antibody in that case was directed against a glycolipid present in mature oligodendrocytes and myelin but not in precursor cells. We now report the effects of implanting a different hybridoma (O4) that secretes IgM antibodies directed against sulfatide, a constituent not only of mature myelin and oligodendrocytes but also of late precursor cells, in order to determine whether this hybridoma too would generate focal demyelination and would, in addition, block remyelination. Our results show that focal plaques of demyelination indeed appear after O4 implantation, and that remyelination does occur, but only in cases where the hybridoma cells have degenerated, probably through host rejection. The occurrence of remyelination suggests that oligodendrocyte precursor cells are capable of migrating in rapidly from adjacent areas or that early precursors, not yet expressing sulfatide, remain undamaged within the lesions. In cases where intact hybridoma cells persist at lesion sites, remyelination does not occur. Failure of remyelination in this model thus appears to result from the continuing presence of antimyelin antibodies rather than from depletion of oligodendrocyte precursors.
Similar content being viewed by others
References
ABRAMSKY, O., LISAK, R. P., SILBERBERG, D. H. & PLEASURE, D. E. (1977) Antibodies to oligodendroglia in patients with multiple sclerosis. New England Journal of Medicine 297, 1207–1211.
BANSAL, R., WARRINGTON, A. E., GARD, A. L., RANSCHT, B. & PFEIFFER, S. E. (1989) Multiple and novel specificities of monoclonal antibodies O1, O4, and R-mAb used in the analysis of oligodendrocyte development. Journal of Neuroscience Research 24, 548–557.
BORNSTEIN, M. B. & RAINE, C. S. (1976) The initial structural lesion in serum-induced demyelination in vitro. Laboratory Investigation 35, 391–401.
BOSTOCK, H. & SEARS, T. S. (1978) The internodal axon membrane: Electrical excitability and continuous conduction in segmental demyelination. Journal of Physiology (London) 280, 273–301.
COMPSTON, D. A., MORGAN, B. P., CAMPBELL, A. K., WILKINS, P., COLE, G., THOMAS, N. D. & JASANI, B. (1989) Immunocytochemical localization of the terminal complement complex in multiple sclerosis. Neuropathology and Applied Neurobiology 15, 307–316.
DUGANDZIJA-NOVAKOVIC, S., KOSZOWSKI, A. G., LEVINSON, S. R. & SHRAGER, P. (1995) Clustering of Na+ channels and node of Ranvier formation in remyelinating axons. Journal of Neuroscience 15, 492–503.
FRANKLIN, R., GILSON, J. M. & BLAKEMORE, W. F. (1997) Local recruitment of remyelinating cells in the repair of demyelination in the central nervous system. Journal of Neuroscience Research 50, 337–344.
FRICK, E. & STICKL, H. (1976) The pathogenesis of multiple sclerosis. Cytotoxic antibodies against myelin sheath tissue in multiple sclerosis. Fortschrift f ¨ur Medizin 94, 1019–1024.
HARTUNG, H. P. & RIECKMANN, P. (1997) Pathogenesis of immune-mediated demyelination in the CNS. Journal of Neural Transmission Suppl. 50, 173–181.
HILLE, B. (2001) Ion Channels of Excitable Membranes, 3rd edition pp. 456–457. Sinauer: Sunderland.
KEIRSTEAD, H. S., HUGHES, H. C. & BLAKEMORE, W. F. (1998) A quantifiable model of axonal regeneration in the demyelinated adult rat spinal cord. Experimental Neurology 151, 303–313.
KOJIMA, K., WEKERLE, H., LASSMANN, H., BERGER, T. & LININGTON, C. (1997) Induction of experimental autoimmune encephalomyelitis by CD4+T cells specific for an astrocyte protein, S100 beta. Journal of Neural Transmission Suppl. 49, 43–51.
KOLES, Z. J. & RASMINSKY, M. (1972) A computer simulation of conduction in demyelinated nerve fibres. Journal of Physiology (London) 227, 351–364.
LANG, E. & ROSENBLUTH, J. (2003) Role of myelination in the development of a uniform olivocerebellar conduction time. Journal of Neurophysiology 89, 2259–2270.
LASSMANN, H., BRUNNER, C., BRADL, M. & LININGTON, C. (1988) Experimental allergic encephalomyelitis: The balance between encephalitogenic T lymphocytes and demyelinating antibodies determines size and structure of demyelinated lesions. Acta Neuropathologica (Berlin) 2, 566–576.
LININGTON, C. & LASSMANN, H. (1989) Immunohistochemical localization of terminal complement complex C9 in EAE. Acta Neuropathologica (Berlin) 79, 78–85.
OZAWA, K., SAIDA, T., SAIDA, K., NISHITANI, H. & KAMEYAMA, M. (1989) In vivo CNS demyelination mediated by anti-galactocerebroside antibody. Acta Neuropathologica (Berlin) 7, 621–628.
PETERS, A., PALAY, S. L. & WEBSTER, H. deF. (1991) The Fine Structure of the Nervous System, 3rd edition, pp. 262–265. New York: Oxford.
PIDDLESDEN, S., LASSMANN, H., ZIMPRICH, F., MORGAN, B. P. & LININGTON, C. (1993) The demyelinating potential of antibodies to myelin oligodendrocyte protein is related to their ability to fix complement. American Journal of Pathology 143, 555–564.
PRINEAS, J. W. (1985) The neuropathology of multiple sclerosis. In Handbook of Clinical Neurology. Vol. 3, Demyelinating Diseases. (edited by KOESTER, J. C.) pp. 213–257. New York: Elsevier.
RAINE, C. S. & CROSS, A. H. (1989) Axonal dystrophy as a consequence of long-term demyelination. Laboratory Investigation 60, 714–725.
RANSCHT, B., WOOD, P. M. & BUNGE, R. P. (1987) Inhibition of in vitro peripheral myelin formation by monoclonal anti-galactocerebroside. Journal of Neuroscience 7, 2936–2947.
ROSENBLUTH, J., LIU, Z., GUO, D. & SCHIFF, R. (1994) Inhibition of CNSmyelin development in vivo by implantation of anti-GalC hybridoma cells. Journal of Neurocytology 23, 699–707.
ROSENBLUTH, J. & MOON, D. (2002) Dysmyelnation induced in vitro by IgM antisulfatide and antigalactocerebroside monoclonal antibodies. Journal of Neuroscience Research 71, 104–109.
ROSENBLUTH, J., SCHIFF, R., LIANG, W. L., DOU, W. K. & MOON, D. (1999) Antibody-mediated demyelination: Focal spinal cord lesions induced by implantation of an IgM antigalacterocerebroside-secreting hybridoma. Journal of Neurocytology 28, 397–416.
SAIDA, T., SAIDA, K. & SILBERBERG, D. H. (1979) Demyelination produced by exprimental allergic neuritis serum and anti-galactocerebroside antiserum in CNS cultures. An ultrastructural study. Acta Neuropathologica (Berlin) 48, 19–25.
SCHIFF, R., ROSENBLUTH, J., DOU, W. K., LIANG, W. L. & MOON, D. (2002) Distribution and morphology of transgenic mouse oligodendroglial-lineage cells following transplantation into normal and myelin-deficient rat CNS. Journal of Comparative Neurology 446, 46–57.
SOLDAN, M. M., WARRINGTON, A. E., BIEBER, A. J., CIRIC, B., VAN KEULEN, V., PEASE, L. R. & RODRIGUEZ, M. (2003) Remyelination-promoting antibodies activate distinct Ca2+ influx pathways in astrocytes and oligodendrocytes: Relationship to the mechanism of myelin repair. Molecular and Cellular Neuroscience 22, 14–24.
SOMMER, I. & SCHACHNER, M. (1981) Monoclonal antibodies (O1 to O4) to oligodendrocyte cell surfaces: An immunocytological study in the central nervous system. Developmental Biology 83, 311–327.
STORCH, M. K., PIDDLESDEN, S., HALTIA, M., LIVANAINEN, M., MORGAN, P. & LASSMANN, H. (1998) Multiple sclerosis: In situ evidence for antibodyand complement-mediated demyelination. Annals of Neurology 43, 465–476.
STYS, P. K., WAXMAN, S. G. & RANSOM, B. R. (1991) Na(+)-Ca2+ exchanger mediates Ca2+ influx during anoxia in mammalian central nervous system white matter. Annals of Neurology 30, 375–380.
TAO-CHENG, J. H. & ROSENBLUTH, J. (1982) Development of nodal and paranodal membrane specializations in amphibian peripheral nerves. Brain Research 255, 577–594.
TRAPP, B. D., PETERSON, B. S., RANSOHOFF, R. M., RUDICK, R., MORK, S. & BO, L. (1998) Axonal transection in the lesions of multiple sclerosis. New England Journal of Medicine 338, 278–285.
WAXMAN S. G. (1977) Conduction in myelinated, unmyelinated and demyelinated fibers. Archives of Neurology 34, 585–589.
YOUNG, W., ROSENBLUTH, J., WOJAK, J. C., SAKATANI, K. & KIM, H. (1989) Extracellular potassium activity and axonal conduction in spinal cord of the myelin-deficient mutant rat. Experimental Neurology 106, 41–51.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Rosenbluth, J., Schiff, R., Liang, WL. et al. Antibody-mediated CNS demyelination II. Focal spinal cord lesions induced by implantation of an IgM antisulfatide-secreting hybridoma. J Neurocytol 32, 265–276 (2003). https://doi.org/10.1023/B:NEUR.0000010085.91976.a6
Issue Date:
DOI: https://doi.org/10.1023/B:NEUR.0000010085.91976.a6