Abstract
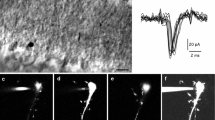
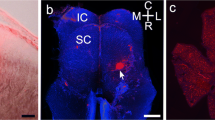
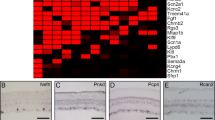
To match a neuron's morphology with its expression of a particular protein, it is useful to first identify the cell by immunostaining and then inject it with fluorescent dye. Such targeted injection cannot be performed with a hydrophilic dye (such as Lucifer yellow) because the neuron, once rendered porous to antibodies, does not retain it. But a lipophilic dye (such as DiI) injected iontophoretically into the soma forms a crystal and is thereby trapped. From this intracellular depot dye diffuses into the cell membrane to reveal the detailed morphology. We have used this strategy to identify the morphology of a GABAergic retinal bipolar cell and several types of GABAergic amacrine cell. In addition, we demonstrate probable connections from a narrow-field, GABAergic amacrine cell to the OFF brisk-transient ganglion cell. Finally, we show that the strategy works in the cortical slice, showing a layer IV cell immunostained for parvalbumin to be a “nest basket cell”.
Similar content being viewed by others
References
CALKINS, D. J., TSUKAMOTO, Y. & STERLING, P. (1998) Microcircuitry and mosaic of a blue/yellow ganglion cell in the primate retina. Journal of Neuroscience 18, 3373–3385.
CALLAWAY, E. M. (2003) Cell types and local circuits in primary visual cortex of the macaque monkey. In The Visual Neurosciences (edited by CHALUPA, L. M. & WERNER, J. S.). Cambridge, MA: MIT Press.
CHUN, M.-H. & WÄSSLE, H. (1989) GABA-likeimmunoreactivity in the cat retina: Electron microscopy. Journal of Comparative Neurology 279, 55–67.
COHEN, E. & STERLING, P. (1990) Demonstration of cell types among cone bipolar neurons of cat retina. Philos. Trans. R. Soc. Lond. B 330, 305–321.
DEMB, J. B., ZAGHLOUL, K., HAARSMA, L. & STERLING, P. (2001) Bipolar cells contribute to nonlinear spatial summation in the brisk transient (Y) ganglion cell in mammalian retina. Journal of Neuroscience 21, 7447–7454.
DOWLING, J. E. & BOYCOTT, B. B. (1966) Organization of the primate retina: Electron microscopy. Proceedings of the Royal Society (London) B 166, 80–111.
EINSTEIN, G., DAVIS, T. L. & STERLING, P. (1987) Pattern of lateral geniculate synapses on neuron somata in layer IV of the cat striate cortex. Journal of Comparative Neurology 260, 76–86.
FREED, M. A. & STERLING, P. (1988) The ON-alpha ganglion cell of the cat retina and its presynaptic cell types. Journal of Neuroscience 8, 2303–2320.
GAN, W.-B., GRUTZENDLER, J., WONG, W. T., WONG, R. O. L. & LICHTMAN, J. W. (2000) Multicolor “Di-Olistic” labeling of the nervous system using lipophilic dye combinations. Neuron 27, 219–225.
GAN, W. B., BISHOP, D. L., TURNEY, S. G. & LICHTMAN, J. W. (1999) Vital imaging and ultrastructural analysis of individual axon terminals labeled by iontophoretic application of lipophilic dye. Journal of Neuroscience Methods 93, 13–20.
HONIG, M. G. & HUME, R. I. (1989) Dil and DiO: Versatile fluorescent dyes for neuronal labelling and pathway tracing. Trends in Neurosciences 12, 333–335.
HUGHES, A. & VANEY, D. I. (1980) Coronate cells: Displaced amacrines of the rabbit retina? Journal of Comparative Neurology 189, 169–189.
KAO, Y.-H., LASSOVÁ, L., STERLING, P. & VARDI, N. (2004) Evidence that two types of retinal bipolar cell use both glutamate and GABA. (Submitted).
KOLB, H. (1979) The inner plexiform layer in the retina of the cat: Electron microscopic observations. Journal of Neurocytology 8, 295–329.
KOLB, H., NELSON, R. & MARIANI, A. (1981) Amacrine cells, bipolar cells and ganglion cells of the cat retina: A Golgi study. Vision Research 21, 1081–1114.
LUKAS, J. R., AIGNER, M., DENK, M., HEINZL, H., BURIAN, M. & MAYR, R. (1998) Carbocyanine postmortem neuronal tracing. Influence of different parameters on tracing distance and combination with immunocytochemistry. Journal of Histochemistry & Cytochemistry 46, 901–910.
MACNEIL, M. A. & MASLAND, R. H. (1998) Extreme diversity among amacrine cells: Implications for function. Neuron 20, 971–982.
MASLAND, R. H. (2001) The fundamental plan of the retina. Nature Neuroscience 4, 877–886.
MCBAIN, C. J. & FISAHN, A. (2001) Interneurons unbound. [Review] [149 refs]. Nature Reviews Neuroscience 2, 11–23.
ROSKA, B. & WERBLIN, F. (2001) Vertical interactions across ten parallel, stacked representations in the mammalian retina. Nature 410, 583–587.
SANDELL, J. H. & MASLAND, R. H. (1988) Photoconversion of some fluorescent markers to a diaminobenzidine product. Journal of Histochemistry and Cytochemistry 36, 555–559.
SOLNICK, B., DAVIS, T. L. & STERLING, P. (1984) Numbers of specific types of neuron in layer IVab of cat striate cortex. Proceedings of the National Academy of Sciences USA 81, 3898–3900.
STERLING, P. (1983) Microcircuitry of the cat retina. Annual Review of Neurosciences 6, 149–185.
STERLING, P. (2003) Howretinal circuits optimize the transfer of visual information. In The Visual Neurosciences (edited by CHALUPA, L. M. & WERNER, J. S.) pp. 243–268. Cambridge, MA: MIT Press.
VANEY, D. I. (1980) A quantitative comparison between the ganglion cell population and axonal outflows of the visual streak and periphery in rabbit retina. Journal of Comparative Neurology 189, 215–233.
VANEY, D. I. (1990) The mosaic of amacrine cells in mammalian retina. In Progress in Retinal Research (edited by OSBORNE, N. & CHADER, J.) pp. 49–100. Oxford, UK: Pergamon Press.
VARDI, N. & AUERBACH, P. (1995) Specific cell types in cat retina express different forms of glutamic acid decarboxylase. Journal of Comparative Neurology 351, 374–384.
WANG, Y., GUPTA, A., TOLEDO-RODRIGUEZ, M., WU, C. Z. & MARKRAM, H. (2002) Anatomical, physiological, molecular and circuit properties of nest basket cells in the developing somatosensory cortex. Cerebral Cortex 12, 395–410.
ZAGHLOUL, K., BOAHEN, K. & DEMB, J. B. (2003) Different circuits forONandOFFretinal ganglion cells cause different contrast sensitivities. Journal of Neuroscience 23, 2645–2654.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kao, YH., Sterling, P. Matching neural morphology to molecular expression: Single cell injection following immunostaining. J Neurocytol 32, 245–251 (2003). https://doi.org/10.1023/B:NEUR.0000010083.03446.78
Issue Date:
DOI: https://doi.org/10.1023/B:NEUR.0000010083.03446.78