Abstract
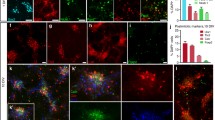
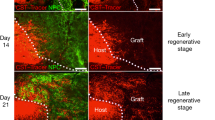
Peripheral nerve grafts in the neostriatum promote axonal regeneration from restricted classes of CNS neuron, principally cells in the substantia nigra pars compacta (SNpc) and striatal cholinergic interneurons. We have examined the molecular responses of CNS neurons induced to regenerate axons by tibial nerve grafting to the neostriatum of adult rats. Brain sections were probed for mRNAs for the transcription factor c-jun, and the cell recognition molecule CHL1, or immunoreacted for TrkA or p75, 1 day to 29 weeks after grafting (dpo; wpo). In unoperated rats, scattered neurons throughout the neostriatum showed weak signals for CHL1 mRNA and slightly stronger signals for c-jun mRNA. Cells of similar appearance strongly expressed TrkA but possessed little p75. By 1 dpo, many neostriatal neurons of various sizes and GFAP + glial cells near the host/graft interface had upregulated CHL1 mRNA, c-jun mRNA and p75. Most of the larger (20–25 μm diameter) CHL1 mRNA+ cells were also TrkA+, indicating that they were NGF-sensitive cholinergic interneurons. From two weeks postgrafting, high levels of CHL1 and c-jun mRNAs and p75 in the neostriatum were confined to a few presumptive cholinergic interneurons; p75+ cells were also TrkA+ and were larger than TrkA+ neurons on the contralateral side. Retrograde labelling showed that most p75+ and some TrkA+ neurons regenerated axons through the graft. Neurons in the SNpc showed a moderate to strong signal for CHL1 mRNA, weaker signal for c-jun mRNA, and no p75 or TrkA. Some SNpc cells upregulated c-jun mRNA after graft implantation, although they did not upregulate CHL1 mRNA, p75 or TrkA. Since neostriatal neurons which regenerate axons into grafts express receptors for NGF, and grafts mimic the effects of NGF treatment on these cells, sensitivity to graft-derived NGF may be a determinant of their high regenerative capacity. The finding that c-jun and CHL1 are consistently expressed by CNS neurons induced to regenerate their axons strongly supports the idea that these molecules are directly involved in axonal regeneration.
Similar content being viewed by others
References
AGUAYO, A. J. (1985) Axonal regeneration from Listinjured neurons in the adult mammalian central nervous system. In Synaptic Plasticity (edited by COTMAN, C. W.) pp. 457–484. New York: The Guilford Press.
ANDERSON, P. N. & LIEBERMAN, A. R. (1999) Intrinsic determinants of differential axonal regeneration by adult mammalian CNS neurons. In Degeneration and Regeneration in the Nervous System (edited by SAUNDERS, N. R. & DZIEGIELEWSKA, K. M.) pp. 53–75. Harwood Academic Press.
ANDERSON, P. N., CAMPBELL, G., ZHANG, Y. & LIEBERMAN, A. R. (1998) Cellular and molecular correlates of the regeneration of adult mammalian CNS axons into peripheral nerve grafts. Progress in Brain Research 117, 211–232.
AUGOOD, S. J., ARBUTHNOTT, G. W. & EMSON, P. C. (1995) Identified cholinergic neurones in the adult rat brain are enriched in GAP-43 mRNA: A double in situ hybridisation study. Journal of Chemical Neuroanatomy 9, 17–26.
BAMJI, S. X., MAJDAN, M., POZNIAK, C. D., BELLIVEAU, D. J., ALOYZ, R., KOHN, J., CAUSING, C. G. & MILLER, F. D. (1998) The p75 neurotrophin receptor mediates neuronal apoptosis and is essential for naturally occurring sympathetic neuron death. Journal of Cell Biology 140, 911–923.
BARTSCH, S., BARTSCH, U., DORRIES, U., FAISSNER, A., WELLER, A., EKBLOM, P. & SCHACHNER, M. (1992) Expression of tenascin in the developing and adult cerebellar cortex. Journal of Neuroscience 12, 736–749.
BENFEY, M., BUNGER, U. R., VIDAL-SANZ, M., BRAY, G. M. & AGUAYO, A. J. (1985) Axonal regeneration from GABAergic neurons in the adult rat thalamus. Journal of Neurocytology 14, 279–296.
BERRY, M., REES, L. & SIEVERS, J. (1986) Unequivocal regeneration of rat optic nerve axons into sciatic nerve isografts. In Neural Transplantation and Regeneration (edited by DAS, G. D. & WALLACE, R. B.) pp. 63–79. New York: Springer-Verlag.
BERRY, M., REES, L., HALL, S., YIU, P. & SIEVERS, J. (1988) Optic axons regenerate into sciatic nerve isografts only in the presence of Schwann cells. Brain Research Bulletin 20, 223–231.
BOLAM, J. P. (2002) Experimental neuroanatomy, a practical approach. Oxford University Press.
BREDESEN, D. E. & RABIZADEH, S. (1997) p75 NTR and apoptosis: Trk-dependent and Trk-independent effects. Trends in Neuroscience 20, 287–290.
CAMPBELL, G., HOLT, J. K. L., SHOTTON, H. R., ANDERSON, P. N., BAVETTA, S. & LIEBERMAN, A. R. (1999) Spontaneous regeneration after optic nerve injury in adult rat. NeuroReport 10, 3955–3960.
CASTELLANI, V., CHEDOTAL, A., SCHACHNER, M., FAIVRE-SARRAILH, C. & ROUGON, G. (2000) Analysis of the L1-deficient mouse phenotype reveals crosstalk between Sema3A and L1 signaling pathways in axonal guidance. Neuron 27, 237–249.
CHAISUKSUNT, V., CAMPBELL, G., ZHANG, Y., SCHACHNER, M., LIEBERMAN, A. R. & ANDERSON, P. N. (2000a) The cell recognition molecule CHL1 is strongly upregulated by injured and regenerating thalamic neurons. Journal of Comparative Neurology 425, 282–292.
CHAISUKSUNT, V., ZHANG, Y., ANDERSON, P. N., CAMPBELL, G., VAUDANO, E., SCHACHNER, M. & LIEBERMAN, A. R. (2000b) Patterns of expression and distribution of mRNAs for L1, CHL1, c-jun and GAP-43 in identified regenerating neurons of the cerebellum and brainstem of the adult rat. Neuroscience 100, 87–108.
CHAO, M., CASACCIA BONNEFIL, P., CARTER, B., CHITTKA, A., KONG, H. & YOON, S. O. (1998) Neurotrophin receptors: Mediators of life and death. Brain Research Brain Research Reviews 26, 295–301.
CHONG, M. S., WOOLF, C. J., ANDREWS, P., TURMAINE, M., SCHREYER, D. J. & ANDERSON, P. N. (1994) The downregulation of GAP-43 is not responsible for the failure of regeneration in freezekilled nerve grafts in the rat. Experimental Neurology 129, 311–320.
DAVEY, F. & DAVIES, A. M. (1998) TrkB signalling inhibits p75-mediated apoptosis induced by nerve growth factor in embryonic proprioceptive neurons. Current Biology 8, 915–918.
DECHANT, G. & BARDE, Y. A. (2002) The neurotrophin receptor p75(NTR): Novel functions and implications for diseases of the nervous system. Nature Neuroscience 5, 1131–1136.
DOMENICONI, M., CAO, Z., SPENCER, T., SIVASANKARAN, R., WANG, K., NIKULINA, E., KIMURA, N., CAI, H., DENG, K., GAO, Y., HE, Z. & FILBIN, M. (2002) Myelin-associated glycoprotein interacts with the nogo66 receptor to inhibit neurite outgrowth. Neuron 35, 283–290.
DOOLEY, J. M. & AGUAYO, A. J. (1982) Axonal elongation fromcerebellum into peripheral nervous system grafts in the adult rat. Annals of Neurology 12, 221.
FERRI, C. C., MOORE, F. A. & BISBY, M. A. (1998) Effects of facial nerve injury on mouse motor neurons lacking the p75 low-affinity neurotrophin receptor. Journal of Neurobiology 34, 1–9.
FORANDER, P., SODERSTROM S., HUMPEL, C. & STROMBERG, I. (1996) Chronic infusion of nerve growth factor into rat striatum increases cholinergic markers and inhibits striatal neuronal discharge rate. European Journal of Neuroscience 8, 1822–1823.
FRADE, J. M. & BARDE, Y. A. (1998) Nerve growth factor: Two receptors, multiple functions. Bioessays 20, 137–145.
FUJITA, N., KEMPER, A., DUPREE, J., NAKAYASU, H., BARTSCH, U., SCHACHNER, M., MAEDA, N., SUZUKI, K. & POPKO, B. (1998) The cytoplasmic domain of the large myelin-associated glycoprotein isoform is needed for properCNSbut not peripheral nervous system myelination. Journal of Neuroscience 18, 1970–1978.
GAGE, F. H., BATCHELOR, P., CHEN, K. S., CHIN, D., HIGGINS, G. A., KOH, S., DEPUTY, S., ROSENBERG, M. B., FISCHER, W. & BJORKLUND, A. (1989) NGF receptor reexpression and NGF-mediated cholinergic neuronal hypertrophy in the damaged adult neostriatum. Neuron 2, 1177–1184.
HAM, J., BABIJ, C., WHITFIELD, J., PFARR, C. M., LALLEMAND, D., YANIV, M. & RUBIN, L. L. (1995) A c-Jun dominant negative mutant protects sympathetic neurons against programmed cell death. Neuron 14, 927–939.
HANBURY, R., CHARLES, V., CHEN, E. Y., LEVENTHAL, L., ROSENSTEIN, J. M., MUFSON, E. J. & KORDOWER, J. H. (2002) Excitotoxic and metabolic damage to the rodent striatum: Role of the P75 neurotrophin receptor and glial progenitors. Journal of Comparative Neurology 444, 291–305.
HEUMANN, R. (1994) Neurotrophin signalling. Current Opinion in Neurobiology 4, 668–679.
HILLENBRAND, R., MOLTHAGEN, M., MONTAG, D. & SCHACHNER, M. (1999) The close homologue of the neural adhesion molecule L1 (CHL1): Patterns of expression and promotion of neurite outgrowth by heterophilic interactions. European Journal of Neuroscience 11, 813–826.
HOLM, J., HILLENBRAND, R., STEUBER, V., BARTSCH, U., MOOS, M., LUBBERT, H., MONTAG, D. & SCHACHNER, M. (1996) Structural features of a close homologue of L1 (CHL1) in the mouse:Anew member of the L1 family of neural recognition molecules. European Journal of Neuroscience 8, 1613–1629.
HÑLL, M. & BÄHR, M. (1994) Regulation of immediateearly gene expression in rat retinal ganglion cells after axotomy and during regeneration through a peripheral nerve graft. Journal of Neurobiology 25, 92–105.
HUNT, D., MASON, M. R. J., CAMPBELL, G., COFFIN, R. & ANDERSON, P. N. (2002) Nogo receptor mRNA expression in intact and regenerating CNS neurons. Molecular and Cellular Neuroscience 20, 537–552.
JENKINS, R., TETZLAFF, W. & HUNT, S. P. (1993) Differential expression of immediate early genes in rubrospinal neurons following axotomy in rat. European Journal of Neuroscience 5, 203–209.
KOKAIA, Z., ANDSBERG, G., MARTINEZ SERRANO, A. & LINDVALL, O. (1998) Focal cerebral ischemia in rats induces expression of P75 neurotrophin receptor in resistant striatal cholinergic neurons. Neuroscience 84, 1113–1125.
LANG, D., HILLENBRAND, R., ZHANG, Y., ANDERSON, P. N., SCHACHNER, M. & BARTSCH, U. (1997) CHL1, a cell recognition molecule closely related to L1, is expressed at elevated levels upon injury or application of bFGF. Society for Neuroscience Abstracts 23, 1996.
LIU, B. P., FOURNIER, A., GRANDPRE, T. & STRITTMATTER, S. M. (2002) Myelin-associated glycoprotein as a functional ligand for the Nogo-66 receptor. Science 297, 1190–1193.
LUNDBERG, C., WICTORIN, K. & BJORKLUND, A. (1994) Retrograde degenerative changes in the substantia nigra pars compacta following an excitotoxic lesion of the striatum. Brain Research 644, 205–212.
MEIRI, K. F., SAFFELL, J. L., WALSH, F. S. & DOHERTY, P. (1998) Neurite outgrowth stimulated by neural cell adhesion molecules requires growth-associated protein-43 (GAP-43) function and is associated with GAP-43 phosphorylation in growth cones. Journal of Neuroscience 18, 10429–10437.
MICHAEL, G. J., KAYA, E., AVERILL, S., RATTRAY, M., CLARY, D. O. & PRIESTLEY, J. V. (1997) TrkA immunoreactive neurones in the rat spinal cord. Journal of Comparative Neurology 385, 441–455.
MORROW, D. R., CAMPBELL, G., LIEBERMAN, A. R. & ANDERSON, P. N. (1993) Differential regenerative growth ofCNSaxons into tibial and peroneal nerve grafts in the thalamus of adult rats. Experimental Neurology 120, 60–69.
NAUMANN, T., CASADEMUNT, E., HOLLERBACH, E., HOFMANN, J., DECHANT, G., FROTSCHER, M. & BARDE, Y. A. (2002) Complete deletion of the neurotrophin receptor p75NTR leads to long-lasting increases in the number of basal forebrain cholinergic neurons. Journal of Neuroscience 22, 2409–2418.
PAXINOS, G. & WATSON, C. (1986) The rat brain in stereotaxic coordinates. Sydney, Academic Press.
RYE, D. B., SAPER, C. B. & WAINER, B. H. (1984) Stabilization of the tetramethylbenzidine (TMB) reaction product: Application for retrograde and anterograde tracing, and combination with immunohistochemistry. Journal of Histochemistry and Cytochemistry 32, 1145–1153.
SAUER, H. & OERTEL, W. H. (1994) Progressive degeneration of nigrostriatal dopamine neurons following intrastriatal terminal lesions with 6-hydroxydopamine: A combined retrograde tracing and immunocytochemical study in the rat. NeuroReport 59, 401–415.
SCHADEN, H., STUERMER, C. A. & BÄHR, M. (1994) GAP-43 immunoreactivity and axon regeneration in retinal ganglion cells of the rat. Journal of Neurobiology 25, 1570–1578.
SMITH, G. V. & STEVENSON, J. A. (1988) Peripheral nerve grafts lacking viable Schwann cells fail to support central nervous system axonal regeneration. Experimental Brain Research 69, 299–306.
SOBREVIELA, T., CLARY, D. O., REICHARDT, L. F., BRANDABUR, M. M., KORDOWER, J. H. & MUFSON, E. J. (1994) TrkA-immunoreactive profiles in the central nervous system: Colocalization with neurons containing p75 nerve growth factor receptor, choline acetyltransferase, and serotonin. Journal of Comparative Neurology 350, 587–611.
STEININGER, T. L., WAINER, B. H., KLEIN, R., BARBACID, M. & PALFREY, H. C. (1993) High affinity nerve growth factor receptor (Trk) immunoreactivity is localized in cholinergic neurons of the basal forebrain and striatum in the adult rat brain. Brain Research 612, 330–335.
TWISS, J. L., WADA, H. G., FOK, K. S., CHAN, S. D., VERITY, A. N., BAXTER, G. T., SHOOTER, E. M. & SUSSMAN, H. H. (1998) Duration and magnitude of nerve growth factor signaling depend on the ratio of p75LNTR to TrkA. Journal of Neuroscience Research 51, 442–453.
VAN DER ZEE, C. E., ROSS, G. M., RIOPELLE, R. J. & HAGG, T. (1996) Survival of cholinergic forebrain neurons in developing p75NGFR-deficient mICE. Science 274, 1729–1732.
VAUDANO, E., CAMPBELL, G., ANDERSON, P. N., DAVIES, A. P., WOOLHEAD, C., SCHREYER, D. J. & LIEBERMAN, A. R. (1995) The effects of a lesion or a peripheral nerve graft on GAP-43 upregulation in the adult rat brain: An in situ hybridization and immunocytochemical study. Journal of Neuroscience 15, 3594– 3611.
VAUDANO, E., CAMPBELL, G., HUNT, S. P. & LIEBERMAN, A. R. (1998) Axonal injury and peripheral nerve grafting in the thalamus and cerebellum of the adult rat: Upregulation of c-jun and correlation with regenerative potential. European Journal of Neuroscience 10, 2644–2656.
WANG, K. C,. KIM, J. A., SIVASANKARAN, R., SEGAL, R. & HE, Z. (2002) p75 interacts with the Nogo receptor as a co-receptor for Nogo, MAG and OMgp. Nature 420, 74–78.
WOOD, S. J., PRITCHARD, J. & SOFRONIEW, M. V. (1990) Re-expression of nerve growthfactor receptor after axonal injury recapitulates a developmental event in motor neurons: Differential regulation when regeneration is allowed or prevented. European Journal of Neuroscience 2, 650–657.
WOOLHEAD, C. L., ZHANG, Y., LIEBERMAN, A. R., SCHACHNER, M., EMSON, P. C. & ANDERSON, P. N. (1998) Differential effects of autologous peripheral nerve grafts to the corpus striatum of adult rats on the regeneration of axons of striatal and nigral neurons and on the expression of GAP-43 and the cell adhesion molecules N-CAM and L1. Journal of Comparative Neurology 391, 259–273.
YOON, S. O., CASACCIA-BONNEFIL, P., CARTER, B. & CHAO, M. V. (1998) Competitive signaling between TrkA and p75 nerve growth factor receptors determines cell survival. Journal of Neuroscience 18, 3273–3281.
ZAGREBELSKY, M., BUFFO, A., SKERRA, A., SCHWAB, M. E., STRATA, P. & ROSSI, F. (1998) Retrograde regulation of growth-associated gene expression in adult rat Purkinje cells by myelin-associated neurite growth Expression of TrkA, p75, c-jun and CHL1 in the injured striatum 183 inhibitory proteins. Journal of Neuroscience 18, 7912–7929.
ZHANG, Y., CAMPBELL, G., ANDERSON, P. N., MARTINI, R., SCHACHNER, M. & LIEBERMAN, A. R. (1995) Molecular basis of interactions between regenerating adult rat thalamic axons and Schwann cells in peripheral nerve grafts I. Neural cell adhesion molecules. Journal of Comparative Neurology 361, 193– 209.
ZHANG, Y., ROSLAN, R., LANG, D., SCHACHNER, M., LIEBERMAN, A. R. & ANDERSON, P. N. (2000) Expression of CHL1 and L1 by neurons and glia following sciatic nerve and dorsal root injury. Molecular and Cellular Neuroscience 16, 71–86.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Chaisuksunt, V., Campbell, G., Zhang, Y. et al. Expression of regeneration-related molecules in injured and regenerating striatal and nigral neurons. J Neurocytol 32, 161–183 (2003). https://doi.org/10.1023/B:NEUR.0000005601.59097.24
Issue Date:
DOI: https://doi.org/10.1023/B:NEUR.0000005601.59097.24