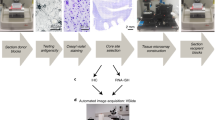
Abstract
Many diseases of the nervous system cause dysfunction by impairing neuronal physiology more than by altering brain anatomy—including age-related cognitive decline, most psychiatric disorders, and even the earliest stages of Alzheimer's disease. The absence of clear anatomical markers makes it difficult to identify targeted cells, which in turn impedes attempts to isolate the pathogenic molecules that cause physiologic disruption. Here we show how brain imaging and microarray can be used as complimentary techniques that together can characterize the cellular and molecular aspects of this class of diseases.
Similar content being viewed by others
References
Gowers, W. R. 1886. A manual of diseases of the nervous system. Churchill, London.
Ribeiro, S., Mello, C. V., Velho, T., Gardner, T. J., Jarvis, E. D., Pavlides, C. 2002. Induction of hippocampal long-term potentiation during waking leads to increased extrahippocampal zif-268 expression during ensuing rapid-eye-movement sleep. J. Neurosci. 22:10914–23.
Koivisto, K., Reinikainen, K. J., Hanninen, T., Vanhanen, M., Helkala, E. L., et al. 1995. Prevalence of age-associated memory impairment in a randomly selected population from eastern Finland. Neurology 45:741–7.
Small, S. A. 2001. Age-related memory decline; current concepts and future directions. Arch. Neurol. 58:360–4.
Loring, D. W. and Papanicolaou, A. C. 1987. Memory assessment in neuropsychology: theoretical considerations and practical utility. J. Clin. Exp. Neuropsychol. 9:340–58.
Zelinski, E. M., Gilewski, M. J., Schaie, K. W. 1993. Individual differences in cross-sectional and 3-year longitudinal memory performance across the adult life span. Psychol. Aging 8:176–86.
Zelinski, E. M., Burnight, K. P. 1997. Sixteen-year longitudinal and time lag changes in memory and cognition in older adults. Psychol. Aging 12:503–13.
Small, S. A., Stern, Y., Tang, M., Mayeux, R. 1999. Selective decline in memory function among healthy elderly. Neurology 52:1392–6.
Jacobs, D. M., Sano, M., Dooneief, G., Marder, K., Bell, K. L., Stern, Y. 1995. Neuropsychological detection and characterization of preclinical Alzheimer's disease [comment] [see comments]. Neurology 45:957–62.
Braak, H., Braak, E. 1996. Evolution of the neuropathology of Alzheimer's disease. Acta. Neurol. Scand. Suppl. 165:3–12.
Esposito, G., Kirkby, B. S., Van Horn, J. D., Ellmore, T. M., Berman, K. F. 1999. Context-dependent, neural system-specific neurophysiological concomitants of ageing: mapping PET correlates during cognitive activation. Brain 122:963–79.
Gomez-Isla, T., Price, J. L., McKeel, D. W., Jr., Morris, J. C., Growdon, J. H., Hyman, B. T. 1996. Profound loss of layer II entorhinal cortex neurons occurs in very mild Alzheimer's disease. J. Neurosci. 16:4491–500.
Gallagher, M., Landfield, P. W., McEwen, B., Meaney, M. J., Rapp, P. R., et al. 1996. Hippocampal neurodegeneration in aging. Science 274:484–5.
Gallagher, M. 1997. Animal models of memory impairment. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 352:1711–7.
Amaral, D. G., Insausti, R. 1990. The hippocampal formation. In the human nervous system., R. Paxinos, ed. San Diego: Academic Press
Zhao, X., Lein, E. S., He, A., Smith, S. C., Aston, C., Gage, F. H. 2001. Transcriptional profiling reveals strict boundaries between hippocampal subregions. J. Comp. Neurol. 441:187–96.
West, M. J., Coleman, P. D., Flood, D. G., Troncoso, J. C. 1994. Differences in the pattern of hippocampal neuronal loss in normal ageing and Alzheimer's disease. Lancet. 344:769–72.
Takahashi, R. H., Milner, T. A., Li, F., Nam, E. E., Edgar, M. A., et al. 2002. Intraneuronal Alzheimer abeta42 accumulates in multivesicular bodies and is associated with synaptic pathology. Am. J. Pathol. 161:1869–79.
D'Andrea, M. R., Nagele, R. G., Wang, H. Y., Lee, D. H. 2002. Consistent immunohistochemical detection of intracellular beta-amyloid42 in pyramidal neurons of Alzheimer's disease entorhinal cortex. Neurosci. Lett. 333:163–6.
Cataldo, A., Rebeck, G. W., Ghetri, B., Hulette, C., Lippa, C., et al. 2001. Endocytic disturbances distinguish among subtypes of Alzheimer's disease and related disorders. Ann. Neurol. 50:661–5.
Selkoe, D. J. 2002. Alzheimer's disease is a synaptic failure. Science 298:789–91.
Sokoloff, L. 1996. Cerebral metabolism and visualization of cerebral activity. Pages 579–602, In Comprehensive human physiology, R. Gregor, U. Windhorst, eds. Springer-Verlag, New York.
Barnes, C. A. 1994. Normal aging: regionally specific changes in hippocampal synaptic transmission. Trends Neurosci. 17:13–8.
Kety, S. 1960. Theory of blood-tissue exchange and its application to measurment of blood flow. Method Med. Res. 8:223.
Siesjo, B. 1978. Brain energy metabolism. Wiley, New York.
Hyder, F., Renken, R., Kennan, R. P., Rothman, D. L. 2000. Quantitative multi-modal functional MRI with blood oxygenation level dependent exponential decays adjusted for flow attenuated inversion recovery (BOLDED AFFAIR). Magn. Reson. Imaging 18:227–35.
Hyder, F., Kida, I., Behar, K. L., Kennan, R. P., Maciejewski, P. K., Rothman, D. L. 2001. Quantitative functional imaging of the brain: towards mapping neuronal activity by BOLD fMRI. NMR Biomed. 14:413–31.
Davis, T. L., Kwong, K. K., Weisskoff, R. M., Rosen, B. R. 1998. Calibrated functional MRI: mapping the dynamics of oxidative metabolism. Proc. Natl. Acad. Sci. USA 95:1834–9.
Cohen, E. R., Ugurbil, K., Kim, S. G. 2002. Effect of basal conditions on the magnitude and dynamics of the blood oxygenation level-dependent fMRI response. J. Cereb. Blood. Flow. Metab. 22:1042–53.
Small, S. A., Nava, A. S., Perera, G. M., Delapaz, R., Stern, Y. 2000. Evaluating the function of hippocampal subregions with high-resolution MRI in Alzheimer's disease and aging [In Process Citation]. Microsc. Res. Tech. 51:101–8.
Mueggler, T., Sturchler-Pierrat, C., Baumann, D., Rausch, M., Staufenbiel, M., Rudin, M. 2002. Compromised hemodynamic response in amyloid precursor protein transgenic mice. J. Neurosci. 22:7218–24.
Bach, M. E., Barad, M., Son, H., Zhuo, M., Lu, Y. F., et al. 1999. Age-related defects in spatial memory are correlated with defects in the late phase of hippocampal long-term potentiation in vitro and are attenuated by drugs that enhance the cAMP signaling pathway. Proc. Natl. Acad. Sci. USA 96:5280–5.
Hsia, A. Y., Masliah, E., McConlogue, L., Yu, G. Q., Tatsuno, G., et al. 1999. Plaque-independent disruption of neural circuits in Alzheimer's disease mouse models. Proc. Natl. Acad. Sci. USA 96:3228–33.
Smith, C. B., Goochee, C., Rapoport, S. I., Sokoloff, L. 1980. Effects of ageing on local rates of cerebral glucose utilization in the rat. Brain 103:351–65.
Gonzalez-Lima, F. 1987. Cytochrome oxidase in neuronal metabolism and Alzheimer's disease. Plenum Press, New York.
Small, S. A. 2003. Microscopic Measurements of Brain Metabolism with MRI; An effective approach for diagnosing Alzheimer's disease and mapping its course. Alzheimer's Disease and Associative Disorders 17:154–161.
Small, S., Wu, E., Bartsch, D., Lacefield, C., DeLaPaz, R., et al. 2000. Imaging physiologic dysfunction of individual hippocampal subregions in humans and genetically modified mice. Neuron. 28:653–64.
Small, S. A., Tsai, W. Y., DeLaPaz, R., Mayeux, R., Stern, Y. 2002. Imaging hippocampal function across the human life span: is memory decline normal or not? Ann. Neurol. 51:290–5.
Colangelo, V., Schurr, J., Ball, M. J., Pelaez, R. P., Bazan, N. G., Lukiw, W. J. 2002. Gene expression profiling of 12633 genes in Alzheimer hippocampal CA1: transcription and neurotrophic factor down-regulation and up-regulation of apoptotic and proinflammatory signaling. J. Neurosci. Res. 70:462–73.
Ginsberg, S. D., Hemby, S. E., Lee, V. M., Eberwine, J. H., Trojanowski, J. Q. 2000. Expression profile of transcripts in Alzheimer's disease tangle-bearing CA1 neurons. Ann. Neurol. 48:77–87.
Loring, J. F., Wen, X., Lee, J. M., Seilhamer, J., Somogyi, R. 2001. A gene expression profile of Alzheimer's disease. DNA Cell. Biol. 20:683–95.
Bahn, S., Augood, S. J., Ryan, M., Standaert, D. G., Starkey, M., Emson, P. C. 2001. Gene expression profiling in the post-mortem human brain—no cause for dismay. J. Chem. Neuroanat. 22:79–94.
Van Deerlin, V., Ginsberg, S. D., Lee, V. M., Trojanowski, J. Q. 2002. The use of fixed human post mortem brain tissue to study mRNA expression in neurodegenerative diseases: applications of microdissection and mRNA amplification. Pages 201–235, in Microarrays for the neurosciences: an essential guide, D. Geschwind, J. Gregg, eds. MIT Press, Boston.
Amaral, D. G. 1993. Emerging principles of intrinsic hippocampal organization. Curr. Opin. Neurobiol. 3:225–9.
Marinkovic, S., Milisavljevic, M., Puskas, L. 1992. Microvascular anatomy of the hippocampal formation. Surg. Neurol. 37:339–49.
Spillane, J. D. 1981. The doctrine of the nerves. Oxford University Press, Oxford.
Chawla, M. K., Barnes, C. A., Rapp, P. R., Pierce, A. L., Small, S. A. 2003. Aging selectively targets the dentate gyrus in Rats, Monkeys, and Humans. 34th annual meeting of the Society for Neuroscience. New Orleans, 2003.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pierce, A., Small, S.A. Combining Brain Imaging with Microarray: Isolating Molecules Underlying the Physiologic Disorders of the Brain. Neurochem Res 29, 1145–1152 (2004). https://doi.org/10.1023/B:NERE.0000023601.50101.7f
Issue Date:
DOI: https://doi.org/10.1023/B:NERE.0000023601.50101.7f