Abstract
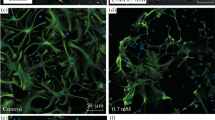
Astrocytes are very sensitive to alterations in the brain environment and respond showing a phenomenon known as astroglial reaction. S100β is an astroglial derived neurotrophic factor, seems to be involved in neuroplasticity. The aim of this work was to study the astrocytic response in rat hippocampus and cerebral cortex after repetitive seizures induced by 3-mercaptopropionic acid (MP) administration. Immunocytochemical studies were performed to analyze GFAP and S100β expression. Both studied areas showed hypertrophied astrocytes with enlarged processes and increased soma size. Astrocyte hyperplasia was observed only in the cerebral cortex. A significant decrease in the astrocytic S100β immunostaining occurs after MP treatment. These results indicate that MP administration induces an astroglial reaction with reduced intracellular S100β level. The observed reduction in astroglial S100β could be related to the release of this factor to the extracellular space, where it may produce neurotrophic or deleterious effects accordingly to the concentration achieved. The mechanism of this remains to be elucidated.
Similar content being viewed by others
references
Walz, W. 1989. Role of glial cells in the regulation of the brain ion microenvironment. Prog. Neurobiol. 33:309–333.
Anderson, C. M. and Swanson, R. A. 2000. Astrocyte glutamate transport: Review of properties, regulation and physiological functions. Glia 32:1–14.
Araque, A., Parpura, V., Sannzgiri, R. P., and Haydon, P. G. 1999. Tripartite synapse: Glia, the unacknowledged partner. TINS. 22:208–215.
Carmignoto, G. 2000. Reciprocal communication system between astrocytes and neurones. Prog. Neurobiol. 62:561–581.
Meldrum, B. S. 1994. The role of glutamate in epilepsy and other CNS disorders. Neurology 44:514–523.
Hinterkeuser, S., Schroder, W., Hager, G., Seifert, G., Blumcke, I., Elger, C. E., Schramm, J., and Steinhauser, C. 2000. Astrocytes in the hippocampus of patients with temporal lobe epilepsy display changes in potassium conductances. Eur. J. Neurosci. 12:2087–2096.
Eng, L. F., Ghirnikar, R. S., and Lee, Y. L. 2000. Glial fibrillary acidic: GFAP-thirty-one years (1969–2000). Neurochem. Res. 25:439–1451.
Brusco, A., Pecci Saavedra, J., García, G., Tagliaferro, P., Evangelista de Duffard, A. M., and Duffard, R. 1997. 2,4-Dichlorophenoxyacetic acid through lactation induces astrogliosis in rat brain. Mol. Chem. Neuropathol. 30:175–185.
Tagliaferro, P., Ramos, A. J. López, E. M., Pecci-Saavedra, J., and Brusco, A. 1997. Neural and astroglial effects of a chronic parachlorophenylalanine-induced serotonin synthesis inhibition. Mol. Chem. Neuropathol. 32:195–211.
Azmitia, E. C., Dolan, K., and Whitaker-Azmitia, P. M. 1990. S100B but not NGF, EGF, insulin or calmodulin is a CNS serotoninnergic growth factor. Brain Res. 516:354–356.
Gomez, L. A., Brusco, A., and Saavedra, J. P 1992. Immunocytochemical study of S-100 positive glial cells in the brainstem and spinal cord of the rat embryo. Int. J. Dev. Neurosci. 8:55–64.
Adami, C., Sorci, G., Blasi, E., Agneletti, A. L, Bistoni, F., and Donato, R. 2001. S100β expression in and effects on microglia. Glia 33:131–142.
Hu, J., Castets, F., Guevara, J. L., and Van Eldik, L. J. 1996. S100β stimulates inducible nitric oxide synthase activity and mRNA levels in rat cortical astrocytes. J. Biol. Chem. 271:2543–2547.
Ramos, A. J., Tagliaferro, P., Lopez, E. M., Pecci Saavedra, J., and Brusco, A. 2000. Neuroglial interactions in a model of parachlorophenylalanine-induced serotonin depletion. Brain Res. 883:1–14.
Sprince, H., Parker, C. M., Josephs, J. A., Jr., and Magazino J. 1969. Convulsant activity of homocysteine and other short-chain mercaptoacids: Protection therefrom. Ann. NY Acad. Sci. 166:323–325.
Girardi, E., Perez Raffo, G., and Rodriguez de Lores Arnaiz, G. 1989. Increase of 5′-nucleotidase activity in some brain subcellular fractions after the administration of the convulsant 3-mercaptopropionic acid. Neurochem. Int. 14:331–335.
Giraldez, L. and Girardi, E. 1998. Modification of 3H-MK801 binding to rat brain NMDA receptors after the administration of a convulsant drug and an adenosine analogue: A quantitative autoradiographic study. Neurochem. Res. 23:1327–1336.
Vanore, G., Giraldez, L., Rodriguez de Lores Arnaiz, G., and Girardi, E. 2001. Seizure activity produces differential changes in adenosine A1 receptors within rat hippocampus. Neuroch. Res. 26:225–230.
De Sarro, G., Ferreri, G., Gareri, P., Russo, E., De Sarro, A., Gitto, R., and Chimirri, A. 2003. Comparative anticonvulsant activity of some 2,3-benzodiazepine derivates in rodents. Pharmacol. Biochem. Behav. 74:595–602.
Rodriguez de Lores Arnaiz, G., Alberici de Canal, M., Robiolo, B., and Mistrorigo de Pacheco, M. 1973. The effect of the convulsant 3-mercaptopropionic acid on enzymes of the γ-aminobutyrate system in the rat cerebral cortex. J. Neurochem. 21:615–623.
Girardi, E. and Rodriguez de Lores Arnaiz, G. 1985. Citrate synthase activity increase in homogenates of the cerebral cortex from rats treated with the convulsant 3-mercaptopropionic acid. Neurochem. Int 7:683–688.
Giraldez, L., Zanetti, F., and Girardi, E. 1999. Striatum adenosine A2 receptors are modified during seizure: Effect of cyclopentyladenosine administration. Neurochem. Res. 24:1219–1225.
Schröder, W., Hinterkeuser, S., Seifert, G., Schramm, J., Jabs, R., Wilkin, G. P., and Steinhäuser, C. 2000. Functional and molecular properties of human astrocytes in acute hippocampal slices obtained from patients with temporal lobe epilepsy. Epilepsia 41(Suppl. 6):S181–S184.
Eng, L. F. and Ghirnikar, R. S. 1994. GFAP and astrogliosis. Brain Pathol. 4:229–237.
Walz, W. and Lang, M. K. 1998. Immunocytochemical evidences for a distinct GFAP-negative subpopulation of astrocytes in the adult rat hippocampus. Neurosci Lett. 257:127–130.
Walz, W. 2000. Controversy surrounding the existence of discrete functional classes of astrocytes in adult gray matter. Glia 31:95–103
Khurgel, M., Switzer, R. C. 3rd., Teskey, G. C., Spiller, A. E., Racine, R. J., and Ivy, G. O. 1995. Activation of astrocytes during epileptogenesis in the absence of neuronal degeneration. Neurobiol. Dis. 2:23–35.
Steward, O., Torre, E. R., Tomasuto, R., and Lothman, E. 1992. Seizures and the regulation of astroglial gene expresion. Epilepsy Res. 7 (Suppl):197–209.
Steward, O. 1994. Electroconvulsive seizures upregulate astroglial gene expression selectively in the dentate gyrus. Brain Res. Mol. Brain. Res. 25:217–224.
Franke, H. and Kittner, H. 2001. Morphological alterations of neurones and astrocytes and changes in emotional behaviour in pentilenetetrazol-kindled rats. Pharmacol. Biochem. Beh. 70:291–303.
Zimmer, I. A., Ennis, M., and Shipley, M. T. 1997. Soman-induced seizures rapidly activate astrocytes and microglia in discrete brain regions. J. Comp. Neurol. 378:482–492.
Garzillo, C. L. and Mello, L. E. 2000. Characterization of reactive astrocytes in the chronic phase of the pilocarpine model of epilepsy. Epilepsia. 43 (Suppl 5):107–109.
Fonseca, C. G., Green, C. R., and Nicholson, L. F. 2002. Upregulation in astrocytic conexin 43 gap junction levels may exarcebate generalized seizures in mesial temporal lobe epilepsy. Brain Res. 929:105–116.
Sohl, G., Guldenagel, M., Beck, H., Teubner, B., Traub, O., Gutierrez, R., Heinemann, U., and Willecke, K. 2000. Expression of connexin genes in hippocampus of kainate-treated and kindled rats under conditions of experimental epilepsy. Brain Res. Mol. Brain Res. 83:44–51.
Bendotti, C., Gugliemetti, F., Tortarolo, M., Samanin, R., and Hirst, W. D. 2000. Differential expression of S100beta and glial fibrillary acidic in the hippocampus after kainic acid-induced lesions and mossy fiber sprouting in adult rat. Exp. Neurol. 161:317–329.
Hu, J., Ferreira, A., and Van Eldik, L. J. 1997. S100β induces neuronal cell death through nitric oxidase release from astrocytes. J. Neurochem. 69:2294–2301.
Donato, R. 1999. Functional roles of S100 proteins, calcium-binding proteins of the EF-hand type. Biochim. Biophys. Acta 1450:191–231.
Sheng, J.G., Mrak, R. E., and Griffin, W. S. T. 1994. S100B protein expression in Alzheimer disease: Potential role in the pathogenesis of neuritic plaques. J. Neurosci. Res. 39:398–404.
Griffin, W. S. T., Yeralam, O., Sheng, J. G., Boop, F. A., Mrak, R. E., Rovnaghi, C. R., Burnett, B. A., Feoktistova, A., and Van Eldik, L. J. 1995. Overexpression of the neurotrophic cytokine S100B in human temporal lobe epilepsy. J. Neurochem. 65:228–240.
Dingledine, R., McBain, C. J., and McNamara, J. O. 1990. Excitatory amino acid receptors in epilepsy. TIPS 11:334–338.
Bradford, H.F. 1995. Glutamate, GABA and epilepsy. Prog. Neurobiol. 47:477–511.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Girardi, E., Ramos, A.J., Vanore, G. et al. Astrocytic Response in Hippocampus and Cerebral Cortex in an Experimental Epilepsy Model. Neurochem Res 29, 371–377 (2004). https://doi.org/10.1023/B:NERE.0000013739.15160.a8
Issue Date:
DOI: https://doi.org/10.1023/B:NERE.0000013739.15160.a8