Abstract
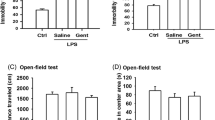
The present study examined effects of the combined administration of naloxone (NX) and indomethacin (IM) on nigrostriatal catecholamines and locomotor activity after intranigral lipopolysaccharide (LPS) injection in Sprague-Dawley rats. NX plus IM was given 3 days after LPS injection; it significantly (P < .05) reversed LPS inflammation on nigrostriatal dopamine (DA) and nigral serotonin (5-HT) and nigral homovanillic acid (HVA)/DA ratio and nigrostriatal 5-hydroxyindoleacetic acid (5-HIAA)/5-HT ratio. It also tended to ameliorate the locomotor hyperactivity. However, NX plus IM given 30 min before LPS could not satisfactorily protect against LPS's damage both biochemically and behaviorally. These results reveal that NX plus IM may protect against LPS on DA, 5-HT, and motor function after LPS injection but not before. Thus it suggests that the combined treatment of NX and IM gives a potent therapy, but not prevention, of LPS-induced inflammation and also protect nigrostriatal dopaminergic and serotoninergic systems against LPS in rats.
Similar content being viewed by others
references
Adolfsson, R., Gottfries, C. G., Roos, B. E., and Winblad, B. J. 1979. Post-mortem distribution of dopamine and homovanillic acid in human brain, variations related to age, and a review of the literature. J. Neural. Transm. 45:81–105.
Hornykiewicz, O. 1989. Aging and neurotoxins as causative factors in idiopathic PD: A critical analysis of the neurochemical evidence. Prog. Neurol. Psychopharmacol. Biol. Psychiatry 13:319–328.
Kish, S. J., Shannak, K., and Hornykiewicz, O. 1988. Uneven pattern of dopamine loss in the striatum of patients with idiopathic Parkinson's disease: Pathophysiologic and clinical implications, New Engl. J. Med. 318:876–880.
Olanow, C. W. and Tatton, W. G. 1999. Etiology and pathogenesis of Parkinson's disease. Annu. Rev. Neurosci. 22:123–144.
Chia, L. G., Liu, S. P., and Lee, E. H. Y. 1992. Differential effects of deprenyl and MPTP on catecholamines and activity in BALB/C mice. Neuroreport 3:777–780.
Gerlach, M., Riederer, P., Przuntek, H., and Youdim, B. H. 1991. MPTP mechanism of neurotoxicity and their implications for Parkinson's disease. Eur. J. Pharmacol. 208:273–286.
Jenner, P., Rupniak, N. M. J., Rose, S., Kelly, E., Kilpatrick, G., Less, A., and Marsden, C. D. 1984. 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced Parkinsonism in the common marmoset. Neurosci. Lett. 50:85–90.
Kordower, J. H., Felten, S. Y., Felten, D. L., and Gash, D. M. 1986. behavioral sequelae following MPTP administration in mice. Pages 413–417, in Markey, S. P., Castagnoli, N. J., Trevor, A. J., and Kopin, I. J. (eds.), MPTP: A Neurotoxin Producing a Parkinsonian Syndrome. Academic Press, New York.
Bronstein, D. M., Perez-Otano, I., Sun, V., Mullis-Sawin, S. B., Chan, J., Wu, G. C., Hudson, P. M., Kong, L. Y., Hong, J. S., and McMillian, M. K. 1995. Glia-dependent neurotoxicity and neuroprotection in mesencephalic culture. Brain Res. 704:112–116.
Liu, B., Du, L., and Hong, J. S. 2000. Naloxone protects rat dopaminergic neurons against inflammatory damage through inhibition of microglia activation and superoxide generation. J. Pharmcol. Exp. Ther. 293:607–617.
Castano, A., Herrera, A. J., Cano, J., and Machado, A. 1998. Lipopolysaccharide intranigral injection induces inflammatory reaction and damage in nigrostriatal dopaminergic system. J. Neurochem. 70:1584–1592.
Hsieh, P. F., Chia, L. G., Ni, D. R., Cheng, L. J., Ho, Y. P., Tzeng, S. F., Chang, M. H., and Hong, J. S. 2002. Behavior, neurochemistry and histology after intranigral lipopolysaccharide injection. Neuroreport 13:277–280.
Colasanti, M., Persichini, T., Di-Pucchio, T., Gremo, F., and Lauro, G. M. 1995. Human ramified microglial cells produce nitric oxide upon Escherichia coli lipopolysaccharide and tumor necrosis factor alpha stimulation. Neurosci. Lett. 200:144–146.
McNaught, K. S. P. and Jenner, P. 2000. Dysfunction of rat forebrain astrocytes in culture alters cytokine and neurotrophic factor release, Neurosci. Lett. 285:61–65.
McGeer, P. L., Itagaki, S., Boyes, B. E., and McGeer E. G. 1988. Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson's and Alzheimer's disease brain. Neurology 38:1285–1291.
McGeer, P. L. and McGeer E. G. 1995. The inflammatory response system of brain: Implications for therapy of Alzeimer and other neurodegenerative diseases. Brain Res. Rev. 21:195–218.
Matyszak, M. K. 1998. Inflammation in the CNS: Balance between immunological privilege and immune response. Prog. Neurobiol. 56:19–35.
Feigenbaum, J. J. and Howard, S. G. 1996. The effect of naloxone on spontaneous and evoked dopamine release in the central and peripheral nervous system. Life Sci. 24:2009–2019.
Liu, B., Jian, J. W., Wilson, B. C., Du, L., Yan, S. N., Wang, J. Y., Wu, J. C., Cao, X. D., and Hong, J. S. 2000. Systemic infusion of naloxone reduces degeneration of rat substantia nigral dopaminergic neurons induced by intranigral injection of lipopolysaccharide, J. Pharmcol. Exp. Ther. 295:125–132.
Lu, X., Bing, X., and Hagg, T. 2000. Naloxone prevents microglia-induced degeneration of dopaminergic substantia nigra neurons in adult rats. Neuroscience 97:285–291.
Kalmar, B., Kittel, A., Lemmens, R., Kornyei, Z., and Madarasz, E. 2001. Cultured astrocytes react to LPS with increased cyclooxygenase activity and phagocytosis. Neurochem. Int. 38:453–461.
Bicego, K. C., Steiner, A. A., Antunes-Rodrigues, J., and Branco, G. S. 2002. Indomethacin impairs LPS-induced behavioral fever in toads. J. Appl. Physiol. 93:512–516.
Montine, K. S., Montine, T. J., Morrow, J. D., Frei, B., Milatovic, D., Eckenstein, F., and Quinn, J. F. 2002. Mouse cerebral prostaglandins, but not oxidative damage, change with age and are responsive to indomethacin treatment. Brain Res. 930:75–82.
Rogers J., Kirby L., Hempelman S., Berry D., McGeer P., Kaszniak A., Zalinski J., Cofield, M., Mansukhani, L., and Willson, P. 1993. Clinical trial of indomethacin in Alzheimer's disease. Neurology 43:1609–1611.
Segal, D. S. and Kuczenski, R. 1974. Tyrosine hydroxylase activity: Regional and subcellular distribution in brain. Brain Res. 68:261–266.
Chia, L. G., Ni, D. R., Cheng, L. J., Kuo, J. S., Cheng, F. C., and Dryhurst, G. 1996. Effects of 1-methyl-4-phenyl-1,2,3,5-tetrahydropyridine and 5,7-dihydroxytryptamine on the locomotor activity and striatal amines in C57BL/6 mice. Neurosci. Lett. 218:67–71.
Masana, M. I., Heyes, M. P., and Mefford, I. N. 1990. Indomethacin prevents increased catecholamine turnover in rat brain following systemic endotoxin challenge. Prog. Neuropsychopharmacol. Biol. Psychiatry 14:609–621.
Yurek, D. M., Deutch, A. Y., Roth, R. H., and Sladek, J. R. 1989. Morphological, neurochemical, and behavioral characterizations associated with the combined treatment of diethyldithiocarbamate and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine in mice. Brain Res. 497:250–259.
Kouhata, S., Kagaya, A., Nakae, S., Nakata, Y., and Yamawaki, S. 2001. Effect of acute lipopolysaccharide administration on (+/-)-1-(2,5-dimethoxy-4-iodophenyl)-2 aminopropane-induced wet dog shake behavior in rats: Comparison with body weight change and locomotor activity. Prog. Neuropsychopharmacol. Biol. Psychiatry 25:395–407.
Chia, L. G., Ni, D. R., Cheng, F. C., Ho, Y. P., and Kuo, J. S. 1999. Intrastriatal injection of 5,7-dihydroxytryptamine decreased 5-HT levels in the striatum and suppressed locomotor activity in C57 BL/6 mice. Neurochem. Res. 24:719–722.
Smee, M. L., Weston, D. F., Skinner, T., and Day, T. 1975. Dose-related effects of central noradrenaline stimulation on behavioural arousal in rats. Psychopharmacol. Commun. 1:123–130.
Suwabe, A., Kubota, M., Niwa, M., Kobayashi, K., and Kanba, S. 2000. Effect of a 5-HT(1A) receptor agonist, flesinoxan, on the extracellular noradrenaline level in the hippocampus and on the locomotor activity of rats. Brain Res. 858:393–401.
You, Z. B., Herrera-Marschitz, M., Nulander, I., Goiny, M., Kehr, J., Ungerstedt, U., and Terenius, L. 1996. Effect of morphine on dynorphin B and GABA release in the basal ganglia of rats. Brain Res. 710:241–248.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, V., Chia, LG., Ni, DR. et al. Effects of the Combined Treatment of Naloxone and Indomethacin on Catecholamines and Behavior After Intranigral Lipopolysaccharide Injection. Neurochem Res 29, 341–346 (2004). https://doi.org/10.1023/B:NERE.0000013736.80749.4b
Issue Date:
DOI: https://doi.org/10.1023/B:NERE.0000013736.80749.4b