Abstract
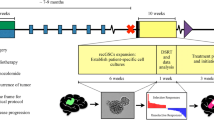
This adjunct to a prospective phase II blinded study of 48 patients with recurrent malignant glioma evaluated the predictive reliability of an extreme drug resistance (EDR) to identify clinical resistance to irinotecan (CPT-11), using fresh tumor biopsies obtained from recurrent patients immediately prior to their first dose of CPT-11 therapy. In vitro tumor response to SN38 (bioactive species of CPT-11 used in the EDR assay) determined prior to treatment was correlated with objective response, time to tumor progression (TTP) and survival following the administration of CPT-11. SN38 activity was tested in 19 of 29 tumors, with 15 of 18 assay results evaluable for correlation with clinical outcomes. In vitro drug resistance was classified as either extreme, intermediate (IDR), or low (LDR). TTP and survival were estimated by the Kaplan–Meier method, and compared using the Mantel–Haenszel log-rank and Fisher's exact test statistics. In vitro tumor response was bifurcated into either EDR (n = 4) or IDR/LDR (n = 11) categories for comparison with outcomes. Results correlated significantly with both TTP and survival. Median TTP for IDR/LDR cases was 3 months versus 6 weeks for EDR cases (log-rank test; p = 0.0288, hazards ratio = 3.06). A 13-week median survival for EDR cases was significantly shorter compared to 38 weeks for IDR/LDR cases (p = 0.029). Further, 100-day survival favored the IDR/LDR cases (Fisher's exact test; p = 0.008). At last follow-up, two of three survivors were patients who had tumors IDR/LDR to SN38. These prospective data support the notion that patients should avoid agents to which their tumor demonstrates EDR.
Similar content being viewed by others
References
Silverberg E, Lubera JA: Cancer statistics. CA Cancer J Clin 39(1): 3-20, 254 and 399 (comments), 1989
Walker AE, Robins M, Weinfeld FD: Epidemiology of brain tumors: the national survey of intracranial neoplasms. Neurology 35: 219-226, 1985
Hochberg FH, Pruitt AA: Assumptions in the radiotherapy of glioblastoma. Neurology 30: 907-911, 1980
Friedman H, Cokgor I, Kerby T, Lawless A, Rich J, Stewart E, Rasheed K, Affronti M, Tourt-Uhlig S, Provenzale J, McLendon R, Colvin O, Bigner D, Malczyn J, Friedman A, Miller L: Phase I trial of CPT-11 plus BCNU in malignant glioma. Proc Am Soc Clin Oncol 18: 150a, A577, 1999
Colvin OM, Cokgor I, Ashley DM, Kerby T, Arbuck S, Malczyn J, Miller L, Cloughesy T, Houghton PJ, Rich J, Friedman AH, Friedman HS: Irinotecan treatment of adults with recurrent or progressive malignant glioma. Proc Am Soc Clin Oncol 17: 387a, A1493, 1998
Friedman HS, Petros WP, Friedman AH, Schaaf LJ, Kerby T, Lawyer J, Parry M, Houghton PJ, Lovell S, Rasheed K, Cloughesy T, Stewart ES, Colvin OM, Provenzale JM, McLendon RE, Bigner DD, Cokgor I, Haglund M, Rich J, Ashley D, Malczyn J, Elfring GL, Miller LL: Irinotecan therapy in adults with recurrent or progressive malignant glioma. J Clin Oncol 17: 1516-1525, 1999
Reid J, Buckner J, Schaaf L, Novotny P, Wright K, Kimmel D, Miller L: Pharmacokinetics of irinotecan (CPT-11) in recurrent glioma patients: results of an NCCTG phase II trial. Proc Am Soc Clin Oncol 18: 141a, A540, 1999
Filka E, Nelson G, Friedman H, Kabbinavar F, Miller L, Menco H, Cloughesy T: Intrapatient dose escalation of irinotecan in patients with recurrent malignant glioma receiving anticonvulsants. Proc Am Soc Clin Oncol 18: 144a, A552, 1999
Rothenberg ML, Kuhn JG, Burris HA III, Nelson J, Eckardt JR, Tristan-Morales M, Hilsenbeck SG, Weiss GR, Smith LS, Rodriguez GI et al.: Phase I and pharmacokinetic trial of weekly CPT-11. J Clin Oncol 11(11): 2194-2204, 1993
de Forni M, Bugat R, Chabot GG, Culine S, Extra JM, Gouyette A, Madelaine I, Marty ME, Mathieu-Boue A: Phase I and pharmacokinetic study of the camptothecin derivative irinotecan, administered on a weekly schedule in cancer patients. Cancer Res 54(16): 4347-4354, 1994
Negoro S, Fukuoka M, Masuda N, Takada M, Kusunoki Y, Matsui K, Takifuji N, Kudoh S, Niitani H, Taguchi T: Phase I study of weekly intravenous infusions of CPT-11, a new derivative of camptothecin, in the treatment of advanced non-small cell lung cancer. J Natl Cancer Inst 83: 1164-1168, 1991
Abigerges D, Armand JP, Chabot GG, Da Costa L, Fadel E, Cote C, Herait P, Gandia D: Irinotecan (CPT-11) high-dose escalation using intensive high-dose loperamide to control diarrhea. J Natl Cancer Inst 86: 446-449, 1994
Abigerges D, Chabot GG, Armand JP, Herait P, Gouyette A, Gandia D: Phase I and pharmacologic studies of the camptothecin analogue irinotecan administered every 3 weeks in cancer patients. J Clin Oncol 13: 210-221, 1995
Masuda N, Fukuoka M, Kusunoki Y, Matsui K, Takifuji N, Kudoh S, Negoro S, Nishioka M, Nakagawa K, Takada M: CPT-11: a new derivative of camptothecin for the treatment of refractory or relapsed small-cell lung cancer. J Clin Oncol 10: 1225-1229, 1992
Ohno R, Okada K, Masaoka T, Kuramoto A, Arima T, Yoshida Y, Ariyoshi H, Ichimaru M, Sakai Y, Oguro M et al.: An early phase II study of CPT-11: a new derivative of camptothecin, for the treatment of leukemia and lymphoma. J Clin Oncol 8: 1907-1912, 1990
Tsuda H, Takatsuki K, Ohno R, Masaoka T, Okada K, Shirakawa S, Ohashi Y, Ota K: Treatment of adult T-cell leukemia-lymphoma with irinotecan hydrochloride (CPT-11). CPT-11 study group on hematological malignancy. Br J Oncol 70(4): 771-774, 1994
Ptiot HC: US pivotal studies of irinotecan in colorectal carcinoma. Oncology 12(8 Suppl. 6): 48-53, 1998
Rothenberg ML, Eckardt JR, Kuhn JG, Burris HA III, Nelson J, Hilsenbeck SG, Rodriguez GI, Thurman AM, Smith LS, Eckhardt SG, Weiss GR, Elfring GL, Rinaldi DA, Schaaf LJ, Von Hoff DD: Phase II trial of irinotecan in patients with progressive or rapidly recurrent colorectal cancer. J Clin Oncol 14: 1128-1135, 1996
Pitot HC, Wender DB, O'Connell MJ, Schroeder G, Goldberg RM, Rubin J, Mailliard JA, Knost JA, Ghosh C, Kirschling RJ, Levitt R, Windschitl HE: Phase II trial of irinotecan in patients with metastatic colorectal carcinoma. J Clin Oncol 15: 2910-2919, 1997
Gupta E, Lestingi TM, Mick R, Ramirez J, Vokes EE, Ratain MJ: Metabolic fate of irinotecan in humans: correlation of glucuronidation with diarrhea. Cancer Res 54: 3723-3725, 1994
Takimoto CH, Arbuck S: The camptothecins. In: Chabner BA and Longo DL (eds) Cancer Chemotherapy and Biotherapy. 2nd edn., Lippincott-Raven Publishers, Philadelphia, 1996, pp. 463-484
Hsiang YH, Liu LF: Identification of mammalian DNA topo-I as an intracellular target for the anticancer drug camptothecin. Cancer Res 48: 1722-1726, 1988
Voigt W, Matsui S, Yin MB, Burhans WC, Minderman H, Rustum YM: Topoisomerase-I inhibitor SN-38 can induce DNA damage and chromosomal aberrations independent from DNA synthesis. Anticancer Res 18: 3499-3505, 1998
Sun M, Duann P, Lin CT, Zhang H, Liu LF: Rapid chromatin structural alteration induced by topoisomerase I-mediated DNA damage. Ann NY Acad Sci 922: 340-342, 2000
Mehta RS, Cloughesy T, Parker R, Fruehauf JP: Predictive value of extreme drug resistance assay in patients receiving CPT-11 for recurrent glioma: a prospective trial. Proc Am Soc Clin Oncol 18: 219a, A843, 1999
Kern DM, Weisenthal LM: Highly specific prediction of antineoplastic drug resistance with an in vitro assay using suprapharmacologic drug exposures. J Natl Cancer Inst 82: 582-588, 1990
Mehta R, Bornstein R, Yu I-R, Parker RJ, McLaren CE, Nguyen KP, Li K-T, Fruehauf JP: Breast cancer survival and in vitro tumor response in the extreme drug resistance assay. Breast Cancer Res Treat 66: 225-237, 2001
Holloway RW, Mehta RS, Finkler NJ, Li K-T, McLaren CE, Parker RJ, Fruehauf JP: Association between in vitro platinum resistance in the EDR assay and clinical outcomes for ovarian cancer patients. Gynecol Oncol 87: 8-16, 2002
Mechetner E, Kyshtoobayeva A, Zonis S, Kim H, Stroup R, Garcia R, Parker RJ, Fruehauf JP: Levels of multidrug resistance (MDR1) p-glycoprotein expression by human breast cancer correlate with in vitro resistance to taxol and doxorubicin. Clin Cancer Res 4: 389-398, 1998
Kern DH, Drogemuller CR, Kennedy MC, Hildebrand-Zanki SU, Tanigawa N, Sondak VK: Development of miniaturized, improved nucleic acid-precursor incorporation assay for chemosensitivity testing of human solid tumors. Cancer Res 45: 5436-5441, 1985
Kaplan EL, Meier P: Nonparametric estimation from incomplete observations. J Am Stat Assn 53: 457-481, 1958
Parker RJ, Kyshtoobayeva A, Mehta R, Brem H, Brem S, Vanier V, Barger G, Fruehauf JP: In vitro drug response and prognostic marker profiles in primary brain tumors. Proc Am Assn Cancer Res 41: 257, A1637, 2000
Kaneda N, Nagata H, Furuta T, Yokokura T: Metabolism and pharmacokinetics of the camptothecin analogue CPT-11 in the mouse. Cancer Res 50: 1715-1720, 1990
Fruehauf JP, Bosanquet AG: In vitro determination of drug response: a discussion of clinical applications. PPO Update 7: 1-17, 1993
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Parker, R.J., Fruehauf, J.P., Mehta, R. et al. A Prospective Blinded Study of the Predictive Value of an Extreme Drug Resistance Assay in Patients Receiving CPT-11 for Recurrent Glioma. J Neurooncol 66, 365–375 (2004). https://doi.org/10.1023/B:NEON.0000014549.77646.f6
Issue Date:
DOI: https://doi.org/10.1023/B:NEON.0000014549.77646.f6