Abstract
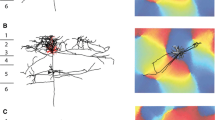
A method based on the retrograde axonal transport of horseradish peroxidase after preliminary (three months) lesioning of the contralateral intermediate nucleus of the cerebellum or lateral vestibular nucleus of Deiters in adult cats demonstrated the formation of new ipsilateral thalamic projections from all three central nuclei of the cerebellum and the nuclei of the vestibular complex.
Similar content being viewed by others
REFERENCES
S. A. Badalyan, J. S. Sarkisyan, and B. I. Pogosyan, “Plastic reorga-nization in the ipsilateral cerebellar-rubral system after lesioning of contralateral intermediate nucleus of the cerebellum,” Biol. Zh. Armenii, 43, No. 10–11, 864–870, (1990).
A. Brodal, F. Val'berg, and O. Pompeano, The Vestibular Nucleus. Connections, Anatomy, Functional Correlations[in Russian], Nauka, Leningrad (1966).
P. Angaut and D. Bowsher, “Ascending projection of the medial cerebellar fastigial nucleus: an experimental study in the cat,” Brain Res., 24, No. 1,49–68 (1970).
A. L. Berman, The Brain Stem of the Cat: A Cytoarchitectonic Atlas with Stereotaxic Coordinates, University of Wisconsin Press, Madison etc. (1968).
C. W. Cotman, M. Nieto-Sampedro, and E. W. Harries, “Synapse replacement in nervous system of adult vertebrates,” Physiol Rev., 61, No. 3, 684–784 (1981).
F. Gomez-Pinilla, J. R. Villablanca, B. J. Sonnier, and M. Levine, “Reorganization of pericruciate cortical projection to the spinal cord and dorsal column nuclei after neonatal or adult cerebral hemi-spherectomy,” Brain Res., 385, No. 2, 343–355 (1986).
A. Gramsbergen and S. Ijkema-Passen, “CNS plasticity after hemicerebellectomy in the young rat. Quantitative relations between aberrant and normal cerebellorubral projections,” Neurosci. Lett., 33, 129–134 (1982).
A. J. Harojan and A. D. Campelone, “A quantitative analysis of the ipsilateral cerebello-thalamic projection following hemicerebellec-tomy in neonatal rats. A retrograde study,” Dev. Brain Res., 26, No.1, 69–79 (1986).
H. H. Jasper and C. A. Aimone-Marsan, A Stereotaxic Atlas of the Diencephalon of the Cat, National Research Council of Canada, Ottawa (1954).
S. Kawaguchi, T. Vamamoto, and A. Samejima, “Electro-physiological evidence for axonal sprouting of cerebellothalamic neurons in kittens after neonatal hemicerebellectomy,” Exptl. Brain Res., 36, No. 1, 21–39 (1979).
N. Kotchabhakdi, E. Rinvik, F. Walberg, and K. Yingchareon, “The vestibulothalamic projections in the cat studied by retrograde axon-al transport of horseradish peroxidase,” Exptl. Brain Res., 40, No.4, 404–418 (1980).
S. K. Leong, “Effects of deafferenting cerebellar on cerebral inputs to the pontine and red nuclei in the albino rat,” Brain Res., 155, No.2,357–361 (1978).
K. H. Lim and S. K. Leong, “Aberrant bilateral projections from the dentate and interposed nuclei in albino rats after neonatal lesions,” Brain Res.,96, No. 2, 306–309 (1975).
R. Maciewicz, B. S. Phipps, L. Bry, and S. M. Higstein, “The vestibulothalamic pathway: contribution of ascending tract of Deiters,” Brain Res., 252, No.1, 1–11 (1982).
M.-M. Mesulam, “Tetramethyl benzidine for HRP neurochemistry: a non-carcinogenic blue reaction product with superior sensitivity for visualizing neural afferents and efferents,” J. Histochem. Cytochem., 26, No. 2, 106–117 (1978).
M. Molinari, M. Bentivoglio, M. Granato, and M. Mincischi, “Increased collateralization of the cerebellothalamic pathway following neonatal hemicerebellectomy,” Brain Res., 372, No. 1, 1–10 (1986).
F. Murakami, H. Katsumaru, K. Saito, and N. Tsukuhara, “A quan-titative study of synaptic reorganization in red nucleus after lesion of the nucleus interpositus of the cat: an electron microscopic study involving intracellular injection of horseradish peroxidase,” Brain Res., 242, No. 1, 41–53 (1982).
C. G. Naus, B. A. Flumerfelt, and A. W. Hrychyshyn, “Topographic specificity of aberrant cerebellorubral projections following neona-tal hemicerebellectomy in the rat,” Brain Res., 309, No. 1, 1–15 (1984).
D. E. Smith and A. J. Castro, “Retrograde changes in Clark's column following neonatal hemicerebellectomy in the rat,” Amer. J. Anat., 156, 533–542 (1979).
S. Snider and W. T. Niemer, A Stereotaxic Atlas of the Cat Brain, University of Chicago Press, Chicago (1961).
J. R. Villablanca and F. Gomez-Pinilla, “Novel crossed corticothala-mic projections after neonatal cerebral hemispherectomy. A quanti-tative autoradiography study in cats,” Brain Res., 410, No.2, 219–231 (1987).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Badalyan, S.A. Plastic Reorganization in the Cerebellothalamic System after Partial Deafferentation of the Ventrolateral Nucleus of the Thalamus. Neurosci Behav Physiol 35, 43–47 (2005). https://doi.org/10.1023/B:NEAB.0000049650.68503.92
Issue Date:
DOI: https://doi.org/10.1023/B:NEAB.0000049650.68503.92