Abstract
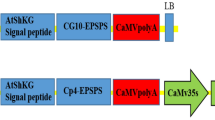
Particle bombardment is a common platform for soybean transformation but tends to cause transgene silencing due to the integration of rearranged or multiple copies of transgenes. We now describe the isolation of a total of 44 independent transgenic soybean plants after transformation by particle bombardment with one of two gene constructs, pHV and pHVS. Both constructs contain the hygromycin phosphotransferase gene (hpt) as a selectable marker and a modified glycinin gene (V3-1) for evaluation of homology-dependent silencing of endogenous glycinin genes; pHVS also contains sGFP(S65T), which encodes a modified form of green fluorescent protein (GFP), as a reporter gene in the flanking region of V3-1. Fluorescence microscopy revealed that the leaves of 8 of the 25 independent transgenic plants obtained with pHVS expressed GFP; most of these GFP-positive plants also contained V3-1 mRNA and an increased glycinin content in their seeds, and they exhibited simple banding patterns on Southern blots that were indicative of a low copy number of each of the three transgenes. In contrast, most of the transgenic plants obtained with pHVS that did not express GFP, as well as most of those obtained with pHV, lacked endogenous glycinin in their seeds and exhibited more complex patterns of transgene integration. The use of a reporter gene such as sGFP(S65T) in addition to an antibiotic resistance gene may thus help to reduce the problem of gene silencing associated with direct DNA transformation systems and facilitate the recovery of transgenic plants that stably express the gene of interest.
Similar content being viewed by others
References
Aragao F.J.L., Sarokin L., Vianna G.R. and Rech E.L. 2000. Selection of transgenic meristematic cells utilizing a herbicidal molecule results in the recovery of fertile transgenic soybean [Glycine max (L.) Merrill] plants at a high frequency. Theor. Appl. Genet. 101: 1-6.
Chiu W.-L., Niwa Y., Zeng W., Hirano T., Kobayashi H. and Sheen J. 1996. Engineered GFP as a vital reporter in plants. Curr. Biol. 6: 325-330.
Dai S., Zheng P., Marmey P., Zhang S., Tian W., Chen S., Beachy R.N. and Fauquet C. 2001. Comparative analysis of transgenic rice plants obtained by Agrobacterium-mediated transformation and particle bombardment. Mol. Breed. 7: 25-33.
Delzer B.W., Somers D.A. and Orf J.H. 1990. Agrobacterium tumefaciens susceptibility and plant regeneration of 10 soybean genotypes in maturity groups 00 to II. Crop Sci. 30: 320-322.
Draper J. and Scott R. 1988. The Isolation of Plant Nucleic Acids. Blackwell Scientific Publications, London, UK.
Elliot A., Campbell J., Dugdale B., Brettell R. and Grof C. 1999. Green-fluorescent protein facilitates rapid in vivo detection of genetically transformed plant cells. Plant Cell Rep. 18: 707-714.
Falco S.C., Guida T., Locke M., Mauvais J., Sanders C., Ward R.T. and Webber P. 1995. Transgenic canola and soybean seeds with increased lysine. Bio/technology 13: 577-582.
Finer J.J. and Nagasawa A. 1988. Development of an embryogenic suspension culture of soybean [Glycine max (L.) Merrill]. Plant Cell Tiss. Org. Cult. 15: 125-136.
Finer J.J. and McMullen M.D. 1991. Transformation of soybean via particle bombardment of embryogenic suspension culture tissue. In Vitro Cell. Dev. Biol. 27: 175-182.
Fu X., Duc L.T., Fontana S., Bong B.B., Tinjuangjun P., Sudhakar D., Twyman R.M., Christou P. and Kohli A. 2000. Linear trans-gene constructs lacking vector backbone sequences generate low-copy-number transgenic plants with simple integration patterns. Transgenic Res. 9: 11-19.
Gamborg O., Miller R. and Ojima K. 1968. Nutrient requirements of suspension cultures of soybean root cells. Exp. Cell Res. 50: 151-158.
Hadi M.Z., McMullen M.D. and Finer J.J. 1996. Transformation of 12 different plasmids into soybean via particle bombardment. Plant Cell Rep. 15: 500-505.
Hansen G. and Wright M. 1999. Recent advances in the transformation of plants. Trends Plant Sci. 4: 226-231.
Harper B., Mabon S., Leffel S., Halfhill M., Richards H., Moyer K. and Stewart J.C. 1999. Green fluorescent protein as a marker for expression of a second gene in transgenic plants. Nature Biotech. 17: 1125-1129.
Haseloff J., Siemering K., Prasher D. and Hodge S. 1997. Removal of a cryptic intron and subcellular localization of green fluorescent protein are required to mark transgenic Arabidopsis plants brightly. Proc. Natl. Acad. Sci. USA 94: 2122-2127.
Herman E.M., Helm R.M., Jung R. and Kinney A.J. 2003. Genetic modification removes an immunodominant allergen from soybean. Plant Physiol. 132: 36-43.
Hinchee M.A.W., Conner-Ward D.V., Newell C.A., McDonnell R.E., Sato S.J., Gasser C.S., Fischhoff D.A., Re D.B., Fraley R.T. and Horsch R.B. 1988. Production of transgenic soybean plants using Agrobacterium-mediated DNA transfer. Bio/technology 6: 915-922.
Jang I.-C., Nahm B. and Kim J.-K. 1999. Subcellular targeting of green fluorescent protein to plastids in transgenic rice plants provides a high-level expression system. Mol. Breed. 5: 453-461.
Jefferson R.A. 1987. Assaying chimeric genes in plants: the GUS gene fusion system. Plant Mol. Biol. Rep. 5: 387-405.
Kim C.S., Kamiya S., Sato T., Utsumi S. and Kito M. 1990. Improvement of nutritional value and functional properties of soybean glycinin by protein engineering. Protein Eng. 3: 725-731.
Kinney A.J., Jung R. and Herman E.M. 2001. Cosuppression of the α subunits of β-conglycinin in transgenic soybean seeds induces the formation of endoplasmic reticulum-derived protein bodies. Plant Cell 13: 1165-1178.
Kitamura Y., Arahira M., Itoh Y. and Fukazawa C. 1990. The complete nucleotide sequence of soybean glycinin A2B1a gene spanning to another glycinin gene A1aB1b. Nucleic Acids Res. 18: 4245.
Klein T.M., Gradziel T., Fromm M.E. and Sanford J.C. 1988. Factors influencing gene delivery into Zea mays cells by high-velocity microprojectiles. Bio/technology 6: 559-563.
Laemmli U.K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 277: 680-685.
Luff B., Pawlowski L. and Bender J. 1999. An inverted repeat triggers cytosine methylation of identical sequences in Arabidopsis. Mol. Cell 3: 505-511.
Matzke A. and Matzke M. 1995. Transinactivation of homologous sequences in Nicotiana tabacum. In: Meyer P. (ed.), Gene Silencing in Higher Plants and Related Phenomena in Other Eukaryotes. Springer, Berlin-Heidelberg-New York, pp. 1-14.
Matzke M., Matzke A.J.M. and Kooter J.M. 2001. RNA: guiding gene silencing. Science 293: 1080-1083.
Maximova S., Dandekar A. and Guiltinan M. 1998. Investigation of Agrobacterium-mediated transformation of apple using green fluorescent protein: high transient expression and low stable transformation suggest that factors other than T-DNA transfer are rate-limiting. Plant Mol. Biol. 37: 549-559.
McCabe D., Swain W.F., Martinell B.J. and Christou P. 1988. Stable transformation of soybean (Glycine max) by particle acceleration. Bio/technology 6: 923-926.
Meyer P. 1995. Understanding and controlling transgene expression. Trends Biotech. 13: 332-337.
Murashige T. and Skoog F. 1962. A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiologia Plantarum 15: 473-497.
Nielsen N.C. 1985. The structure and complexity of the 11S polypeptides in soybean. J. Am. Oil Chem. Soc. 62: 1680-1686.
Nielsen N.C., Dickinson C.D., Cho T.-J., Thanh V.H., Scallon B.J., Fischer R.L., Sims T.L., Drews G.N. and Goldberg R.B. 1989. Characterization of the glycinin gene family from soybean. Plant Cell 1: 313-328.
Ohta S., Mita S., Hattori T. and Nakamura K. 1990. Construction and expression in tobacco of a β-glucuronidase (GUS) reporter gene containing an intron within the coding sequence. Plant Cell Physiol. 31: 805-813.
Owens L.D. and Cress D.E. 1985. Genotypic variability of soybean response to Agrobacterium strains harboring the Ti or Ri plasmids. Plant Physiol. 77: 87-94.
Parrott W.A., All J.N., Adang M.J., Bailey M.A., Boerma H.R. and Stewart C.N.J. 1994. Recovery and evaluation of soybean plants transgenic for a Bacillus thuringiensis var. Kurstaki insecticidal gene. In Vitro Cell. Dev. Biol. 30: 144-149.
Ponappa T., Brzozowski A.E. and Finer J.J. 1999. Transient expression and stable transformation of soybean using the jelly-fish green fluorescent protein. Plant Cell Rep. 19: 6-12.
Sato S., Newell C., Kolacz K., Tredo L., Finer J. and Hinchee M. 1993. Stable transformation via particle bombardment in two different soybean regeneration systems. Plant Cell Rep. 12: 408-413.
Srinivasa Reddy M.S., Dinkins R.D. and Collins G.B. 2003. Gene silencing in transgenic soybean plants transformed via particle bombardment. Plant Cell Rep. 21: 676-683.
Stam M., Mol J. and Kooter J. 1997. The silence of genes in transgenic plants. Ann. Bot. 79: 3-12.
Stewart J.C.N. 2001. The utility of green fluorescent protein in transgenic plants. Plant Cell Rep. 20: 376-382.
Takaiwa F., Katsube T., Kitagawa S., Hisago T., Kito M. and Utsumi S. 1995. High level accumulation of soybean glycinin in vacuole-derived protein bodies in the endosperm tissue of transgenic tobacco seed. Plant Sci. 111: 39-49.
Tian L., Levèe V., Mentag R., Charest P.J. and Sèguin A. 1999. Green fluorescent protein as a tool for monitoring transgenic expression in forest tree species. Tree Physiol. 19: 541-546.
Trick H.N. and Finer J.J. 1998. Sonication-assisted Agrobacterium-mediated transformation of soybean [Glycine max (L.) Merrill] embryogenic suspension culture tissue. Plant Cell Rep. 17: 482-488.
Vaucheret H., Beclin C., Elmayan T., Feuerbach F., Godon C., Morel J.-B., Mourrain P., Palauqui J.-C. and Vernhettes S. 1998. Transgene-induced gene silencing in plants. Plant J. 16: 651-659.
Waterhouse P.M., Wang M.B. and Finnegan E.J. 2001. Role of short RNAs in gene silencing. Trends Plant Sci. 6: 297-301.
Yagasaki K., Kaizuma N., Kitamura K. 1996. Inheritance of glynicin subunits and characterization of glycinin molecules lacking the subunits in soybean (Glycine max (l.) Merr.). Breed. Sci. 46: 11-15.
Yamada T., Teraishi M., Hattori K. and Ishimoto M. 2001. Transformation of azuki bean by Agrobacterium tumefaciens. Plant Cell Tiss. Org. Cult. 64: 47-54.
Zhang Z., Xing A., Staswick P. and Clemente T.E. 1999. The use of glufosinate as a selective agent in Agrobacterium-mediated transformation of soybean. Plant Cell Tiss. Org. Cult. 56: 37-46.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
El-Shemy, H.A., Teraishi, M., Khalafalla, M.M. et al. Isolation of soybean plants with stable transgene expression by visual selection based on green fluorescent protein. Molecular Breeding 14, 227–238 (2004). https://doi.org/10.1023/B:MOLB.0000047772.48746.f4
Issue Date:
DOI: https://doi.org/10.1023/B:MOLB.0000047772.48746.f4