Abstract
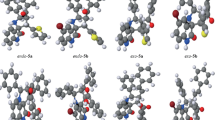
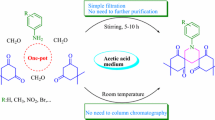
A pseudo four-component reaction is described, leading to the efficient regioselective synthesis of σ symmetric spiro heterobicyclic rings using aldehydes and urea in the presence of cyclic β-diester or β-diamides such as Meldrum's acid or barbituric acid derivatives. The reaction needs no added catalyst and proceeds solvent-free conditions at 80 °C.
Similar content being viewed by others
References
Domling, A. and Ugi, I., Multicomponent reaction with isocyanides, Angew. Chem. Int. Ed., 39 (2000) 3168–3210. (b) Kappe, C.O., High-speed combinatorial synthesis utilizing microwave irradiation, Curr. Opin. Chem. Biol., 6 (2002) 314–320. (c) Domling, A., Recent advances in isocyanide-based multicomponent chemistry, Curr. Opin. Chem. Biol., 6 (2002) 306–313.
Biginelli, P., Gazz. Chim. Ital., 23 (1893) 360.
Kappe, C.O., 100 Years of the Biginelli dihydropyrimidine synthesis, Tetrahedron, 49 (1993) 6937–6963, and references therein. (b) Sabitha, G., Reddy, G.S.K.K., Reddy, K.B. and Yadav, J.S, Vanadium(III) chloride catalyzed Biginelli condensation: Solution phase library generation of dihydropyrimidin-2(1H)-ones, Tetrahedron Lett., 44 (2003) 6497–6499.
Yarim, M., Sarac, S., Kilic, F.S. and Erol, K., Synthesis and in vitro calcium antagonist activity of 4-aryl-7,7-dimethyl/1,7,7-trimethyl-1,2,3,4,5,6,7,8-octahydroquinazoline-2,5-dione derivatives, IL Farmaco, 58 (2002) 17–24.
Byk, G., Gettlieb, H. E., Herscovici, J. and Mirkin, F., New regioselective multicomponent reaction: One pot synthesis of spiro heterobicyclic aliphatic rings, J. Comb. Chem., 2 (2000) 732–735.
Mokrosz, J.L., Paluchowska, M.H., Szneler, E. and Drozdz, B., The effect of aryl substituents in some spiro[2-oxo-4,6-bis(aryl)hexahydropyrimidine-5,5′-barbituric acids], Arch. Pharm. (Weinheim, Germany), 322 (1989) 231–235. (b) Mamaev, V.P. and Borovik, V.P., spiro[2-oxo-4,6-diphenylhexahydropyrimidine-5,5′-barbituric acid], Izobret., Prom. Obraztsy, Tovarnye Znaki, 45 (1968) 24; Chem. Abstr., 69 (1968) 96790. (c) Mamaev, V.P., Borovik, V.P., Gorfinkel, M.I. and Ivanovskaya, L.Yu., Route for the synthesis of spiropyrimidinebarbituric acids, Izv. Akad. Nauk SSSR, Ser. Khim. (1970) 1637–1638; Chem. Abstr., 74 (1971) 22276. (d) Borovik, V.P. and Mamaev, V.P., Synthesis of sulfur-containing spiropyrimidine barbituric acids, Khim. Farm. Zh. 4 (1970) 32–35; Chem. Abstr., 72 (1970) 111411.
Shaabani, A., Bazgir, A. and Teimouri, F., Ammonium chloride-catalyzed one-pot synthesis of 3,4-dihydropyrimidin-2-(1H)-ones under solvent-free conditions, Tetrahedron Lett., 44 (2003) 857–859.
Shaabani, A., Teimouri, M.B. and Bijanzadeh, H.R., One-pot three component condensation reaction in water: An efficient and improved procedure for the synthesis of furo[2,3-d]pyrimidine-2,4(1H,3H)-diones, Tetrahedron Lett., 43 (2002) 9151–9154.
Shaabani, A. and Teimouri, M.B., The reaction of alkyl isocyanides and benzylidene Meldrum's acid derivatives in the presence of water: A one-pot synthesis of 4-(alkylamino)-3-aryl-4-oxobutanoic acids, J. Chem. Research(S), (2002) 433–435.
Shaabani, A., Yavari, I., Teimouri, M.B., Bazgir, A. and Bijanzadeh, H.R., New and efficient synthesis of dialkyl 2-[1-p-nitrophenyl-2-(alkylamino)-2-oxoethyl]malonates, Tetrahedron, 57 (2001) 1375–1378.
Folkers, K. and Johnson, T.B., Researches on pyrimidines. CXXXVI. The mechanism of formation of tetrahydropyrimidines by the Biginelli reaction, J. Am. Chem. Soc., 55 (1933) 3784–3791.
Kappe, C.O., A reexamination of the mechanism of the Biginelli dihydropyrimidine synthesis. Support for an N-acyliminium ion intermediate, J. Org. Chem., 62 (1997) 7201–7204.
Sweet, F. and Fissekis, J.D., On the synthesis of 3,4-dihydropyrimidin-2-(1H)-ones and the mechanism of the Biginelli reaction, J. Am. Chem. Soc., 95 (1973) 8741–8749.
Bigi, F., Carloni, S., Ferrari, L., Maggi, R., Mazzacani, A. and Sartori, G., Clean synthesis in water. Part 2: Uncatalysed condensation reaction of Meldrum's acid and aldehydes, Tetrahedron Lett., 42 (2002) 5203–5205.
Kaup, G., Reza Naim Jamal, M. and Schmeyers, J., Solvent-free Knoevenagel condensation and Michael additions in the solid state and in the melt with quantitative yield, Tetrahedron, 59 (2003) 3753–3760.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shaabani, A., Bazgir, A. & Bijanzadeh, H.R. A reexamination of Biginelli-like multicomponent condensation reaction: One-pot regioselective synthesis of spiro heterobicyclic rings. Mol Divers 8, 141–145 (2004). https://doi.org/10.1023/B:MODI.0000025613.35304.25
Issue Date:
DOI: https://doi.org/10.1023/B:MODI.0000025613.35304.25