Abstract
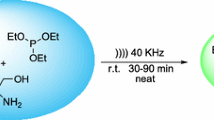
An efficient sonochemical methodology is described for the synthesis of new podands containing substituted dihydropyrimidines.
Similar content being viewed by others
References
Kyu-Sung Jeong, Young Lag Cho, Seung Yup Pyun, Podand ionophores capable of forming cation-binding cavities through intramolecular interaction between the terminal groups, Tetrahedron Lett. 36, (1995) 2827-2830.
Fedorova, O. V., Mordovskoi, G. G., Rusinov, G. L., Zueva, M. N. and Ovchinnikova, I. G., Search of the compounds with tuberculostatic activity in the range of synthetic ionophorespodands, Khim.-pharm. zhurn., 10 (1996) 6-7
Fedorova, O. V., Rusinov, G. L., Mordovskoi, G. G., Zueva, M. N., Kravchenko, M. A., Ovchinnikova, I. G. and Chupakhin O. N., Synthesis and tuberculostatic activity of podands with fluoroquinolone fragment, Khim.-pharm. zhurn., 7 (1997) 21-23
Fedorova, O. V., Rusinov, G. L., Zhidovinova, M. S., Ovchinnikova, I. G. and Chupakhin, O. N., Multicomponent reactions in the synthesis dihydropyrimidine podands, Abstracts of Second International Symposium of Supramolecular Architectures, Kazan, (2002) 154.
Biginelli, P., Aldehyde-urea derivatives of aceto-and oxaloacetic acids, Gazz. Chim. Ital., 23 (1893) 360-413.
Kappe, C. O., Synthesis and aromatization of dihydropyrimidines structurally related to calcium channel modulators of the nifedipine-type, Heterocycles, 45 (1997) 1967-1978.
Kappe, C. O., A reexamination of the mechanism of Biginelli dihydropyrimidine synthesis. Support for an N-acyliminium intermediate, J. Org. Chem., 62 (1997) 7201-7204.
Kappe, C. O., Kumar, D. and Varma, R. S., Microwave-assisted high-speed parallel synthesis of 4-aryl-3,4-dihydropyrimidin-2(1H)-ones using a solventless Biginelli condensation protocol, Synthesis, (1999) 1799-1803.
Stadler, A. and Kappe, C. O., Microwave-mediated Biginelli reactions revisited. On the nature of rate and yield enhancements, J. Chem. Soc. Perkin Trans. 1, (2000) 1363-1368.
Yadav, J. S., Reddy, B. V. S., Reddy, K. B., Raj, K. S. and Prasad, A. R., Ultrasound-accelereted synthesis of 3,4-dihydropyrimidin-2(1H)-ones with ceric ammonium nitrate, J. Chem. Soc., Perkin Trans. 1, (2001) 1939-1941.
Battaglia, L. P. and Corradi, A. B., Synthesis and characterization of some metal complexes with a new nitrogen-oxygen donor macrocyclic ligand, Inorg. Chim. Acta., 42 (1980) 191-196.
Mounetou, E., Legault, J. an Lacroix J., C-Gaudreault R., Antimitotic antitumor agents: synthesis, structure-activity relationships, and biological characterization of N-Aryl-N-(2-chloroethyl)ureas as new selective alkylating agents, J. Med. Chem., 44 (2001) 694-702.
Kobuke, Y., Katsumi, H., Hanji, K. and Horiguchi, K., Macrocyclic ligands composed of tetrahydrofuran for selective transport of monovalent cations through liquid membrane, J. Am. Chem. Soc., 98 (1976) 7414-7419.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhidovinova, M.S., Fedorova, O.V., Rusinov, G.L. et al. Multicomponent sonochemical synthesis of podands. Mol Divers 6, 323–326 (2003). https://doi.org/10.1023/B:MODI.0000006915.32225.3a
Issue Date:
DOI: https://doi.org/10.1023/B:MODI.0000006915.32225.3a