Abstract
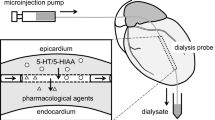
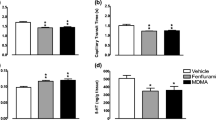
Portal-systemic encephalopathy (PSE) is associated with increased brain turnover of serotonin (5-HT) in vivobut the brain 5-HT output seems to be unaltered. Recent results suggest, however, that an augmented neocortical 5-HT release in experimental chronic PSE may prevail under certain conditions. In the present study, neocortical extracellular 5-HT and 5-hydroxyindoleacetic-3-acid (5-HIAA) levels were measured in portacaval shunted (PCS) rats and sham-operated controls following local administration of p-chloroamphetamine (pCA) and d-fenfluramine (dFEN), two specific 5-HT releasing agents. The basal neocortical extracellular 5-HT concentrations were unaltered and the 5-HIAA levels were elevated in experimental PSE, supporting an unchanged brain 5-HT output despite elevated brain 5-HT metabolism. Perfusion with pCA or dFEN (5 μM; one 20-min pulse) produced marked increases in brain 5-HT release both in PCS and sham-operated rats compared with corresponding basal values. While no difference in the 5-HT response to dFEN administration was seen between sham (5-HT levels increased by 330%) and PCS (500%) rats, a clear difference (p<0.05) in the brain 5-HT output was observed between the two experimental groups following pCA perfusion (sham, 1100% versus PCS, 1470%). These results support our previous contention of an enhanced neocortical 5-HT output in experimental chronic PSE under certain pharmacological conditions.
Similar content being viewed by others
REFERENCES
Adell, A., Sarna, G.S., Hutson, P.H., and Curzon, G. (1989). An in vivo dialysis and behavioural study of the release of 5-HT by p-chloroamphetamine in reserpine-treated rats. Br. J. Pharmacol. 97:206–212.
Bengtsson, F., Bugge, M., Brun, A., Falck, B., Henriksson, K.G., and Nobin, A. (1988). The impact of time after portacaval shunt in the rat on behavior, brain serotonin, and brain and muscle histology. J. Neurolog. Sci. 83:109–122.
Bengtsson, F., Bugge, M., and Nobin, A. (1989). Hepatocerebral dysfunction and brain serotonin. In (R.F. Butterworth, and G. Pomier Layrargues, eds.), Hepatic Encephalopathy: Pathophysiology and Treatment. Humana Press, Clifton, NJ, pp. 355–387.
Bengtsson, F. (1992). Neurotransmission failure in hepatic encephalopathy involving the combined action of different brain tryptophan-related pathology: a speculative synthesis. In (I. Ishiguro, T. Nagatsu, and Y. Nagamura, eds.), Advances in Tryptophan Research 1992. Fujita Health University Press, Toyoake, Japan, pp. 303–308.
Bengtsson, F., and Bergqvist, P.B.F. (1996). Neuropsychiatric implications of brain tryptophan: Perturbations appearing in hepatic encephalopathy. In (G.A. Filippini, C.V.L. Costa, and A. Bertazzo, eds.), Recent Advances in Tryptophan Research. Plenum, New York, NY, pp. 387–395.
Bergqvist, P.B.F., Vogels, B.A.P.M., Bosman, D.K., Maas, M.A.W., Hjorth, S., Chamuleau, R.A.F.M., and Bengtsson, F. (1995). Neocortical dialysate monoamines of rats after acute, subacute, and chronic liver shunt. J. Neurochem. 64:1238–1244.
Bergqvist, P.B.F., Hjorth, S., Apelqvist, G., and Bengtsson, F. (1996a). Acute effects of L-tryptophan on brain extracellular 5-HT and 5-HIAA levels in chronic experimental portal-systemic encephalopathy. Metab. Brain Dis. 11:269–278.
Bergqvist, P.B.F., Hjorth, S., Audet, R., Apelqvist, G., Bengtsson, F., and Butterworth R.F. (1996b). Ammonium acetate challenge in experimental hepatic encephalopathy induces a transient increase of brain 5-HT release in vivo. Eur. Neuropsychopharmacology 6:317–324.
Bergqvist, P.B.F., Hjorth, S., Apelqvist, G., and Bengtsson, F. (1997). Potassium-evoked neuronal release of serotonin in experimental chronic portal-systemic encephalopathy. Metab. Brain Dis. 12:185–194.
Bergqvist, P.B.F., and Bengtsson, F. (1997). Brain tryptophan-related perturbations in hepatic encephalopathy: an update. In (R. Richard, ed.), Current Topics in Neurochemistry, Research Trends, Trivandrum, India, In press.
Bonanno, G., Fassio, A., Severi, P., Ruelle, A., and Raiteri, M. (1994). Fenfluramine releases serotonin from human brain nerve endings by a dual mechanism. J. Neurochem. 63:1163–1166.
de Parada, M.P., Parada, M.A., Pothos, E., and Hoebel B.G. (1995). d-Fenfluramine, but not d-norfenfluramine, uses calcium to increase extracellular serotonin. Life Sci. 56:415–420.
Fuller, R.W., Hines, C.W., and Mills, J. (1965). Lowering of brain serotonin level by chloroamphetamines. Biochem. Pharmacol. 14:483–488.
Garattini, S., Caccia, S., Mennini, T., Samanin, R., Consolo, S., and Ladinsky, H. (1979). Biochemical pharmacology of the anorectic drug fenfluramine: a review. Curr. Med. Res. Opin. 6(suppl1):15–27.
Hjorth, S., and Sharp, T. (1993). In vivo microdialysis evidence for central serotonin1A and serotonin1B autoreceptor blocking properties of the beta adrenoceptor antagonist (−)penbutolol. J. Pharmacol. Exp. Ther. 265:707–712.
Kuhn, D.M., Wolf, W.A., and Youdim, M.B.H. (1985). 5-Hydroxytryptamine release in vivo from a cytoplasmic pool: studies on the 5-HT behavioural syndrome in reserpinized rats. Br. J. Pharmacol. 84:121–129.
Lee, S.H., and Fisher, B. (1961). Portacaval shunt in the rat. Surgery 50:668–672.
Mans, A.M., DeJoseph, M.R., Davis, D.W., Vina, J.R., and Hawkins, R.A. (1990). Early establishment of cerebral dysfunction after portacaval shunting. Am. J. Physiol. 259:E104–E-110.
Mousseau, D.D., and Butterworth, R.F. (1994). Current theories on the pathogenesis of hepatic encephalopathy. Exp. Biol. Med. 206:329–344.
Schröder, R., Müller, O., and Bircher, J. (1985). The portacaval and splenocaval shunt in the normal rat. A morphometric and functional reevaluation. J. Hepatol. 1:107–123.
Swain, M.S., Blei, A.T., Butterworth, R.F., and Kraig, R.P. (1991). Intracellular pH rises and astrocytes swell after portacaval anastomosis in rats. Regulatory Intergrative Comp. Physiol. 30:R1491–R1496.
Trulson, M.E., and Jacobs, B.L. (1976). Behavioral evidence for the rapid release of CNS serotonin by PCA and fenfluramine. Eur. J. Pharmacol. 36:149–154.
Wichems, C.H., Hollingsworth, C.K., and Bennett, B.A. (1995). Release of serotonin induced by 3,4-methylenedioxymethamphetamine (MDMA) and other substituted amphetamines in cultured fetal raphe neurons: further evidence for calcium-independent mechanisms of release. Brain Res. 695:10–18.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bergqvist, P.B., Hjorth, S., Wikell, C. et al. p-Chloroamphetamine- and d-Fenfluramine-Induced Brain Serotonin Release in Experimental Portal-Systemic Encephalopathy. Metab Brain Dis 12, 229–236 (1997). https://doi.org/10.1023/B:MEBR.0000007103.82267.06
Issue Date:
DOI: https://doi.org/10.1023/B:MEBR.0000007103.82267.06