Abstract
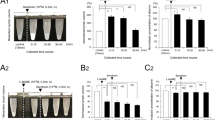
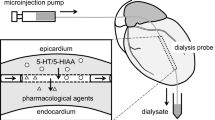
Portal-systemic encephalopathy (PSE) is associated with an increased brain tissue turnover of serotonin (5-HT). Despite increased 5-HT metabolism, brain 5-HT release in rats with a portacaval shunt (PCS) seems to be unaltered. Although this may indicate that the overall 5-HT output is unaltered in PSE, it is also possible that the 5-HT release pattern might be altered in some way. In the present study, the potassium-evoked frontal neocortical release of 5-HT was studied in experimental chronic PSE. KCl (60 mM) produced marked increases in the 5-HT output compared with basal values both in PCS and sham rats. Simultaneously, the KCl challenge resulted in significant elevations in the 5-HT release of PCS compared with sham. In Ca2+-free medium, the difference between PCS and sham rats in the KCl-evoked release of 5-HT was abolished. In the presence of TTX (1 mM), both groups displayed increased extracellular 5-HT levels. Again, a difference with higher amplitude of the 5-HT release in PCS compared with sham was evident. It is concluded that in experimental chronic PSE an augmented neocortical 5-HT release compared with the normal in vivo situation is available. The possible mechanism(s) responsible for the difference in neocortical 5-HT output between PCS and sham-operated rats in response to the KCl-challenge is discussed.
Similar content being viewed by others
REFERENCES
Adam-Vizi, V. (1992). External Ca2+-independent release of neurotransmitters. J. Neurochem. 58:395–405.
Apelqvist, G., Hindfelt, B., Andersson, G., and Bengtsson, F. (1997). Central versus peripheral spontaneous behavioral abnormalities in experimental hepatic encephalopathy. Physiol. Behav. 61:851–856.
Bengtsson, F. (1992). Neurotransmission failure in hepatic encephalopathy involving the combined action of different brain tryptophan-related pathology: a speculative synthesis. In I. Shiguro, T. Nagatsu, and Y. Nagamura, eds.), Advances in Tryptophan Research 1992, Fujita Health University Press, Toyoaka, Japan, pp. 303–308.
Bengtsson, F., Bugge, M., Brun, A., Falck, B., Henriksson, K.G., and Nobin, A. (1988). The impact of time after portacaval shunt in the rat on behavior, brain serotonin, and brain and muscle histology. J. Neurol. Sci. 83:109–122.
Bengtsson, F., Bugge, M., and Nobin, A. (1989). Hepatocerebral dysfunction and brain serotonin. In (R.F. Butterworth, and G. Pomier Layrargues, eds.), Hepatic encephalopathy: Pathophysiology and Treatment. Humana Press, Clifton, NJ, U.S.A., pp. 355–385.
Bengtsson, F., Bugge, M., Johansen, K.H., and Butterworth, R.F. (1991). Brain tryptophan hydroxylation in the portacaval shunted rat: a hypothesis for the regulation of serotonin turnover in vivo. J. Neurochem. 56:1069–1074.
Bengtsson, F., and Bergqvist, P.B.F. (1996). Neuropsychiatric implications of brain tryptophan: Perturbations appearing in hepatic encephalopathy. In (G. Allegri Filippini, C.V.L. Costa, and A. Bertazzo, eds.), Recent Advances in Tryptophan Research, Plenum Publ. Corp., New York, NY, U.S.A., pp. 387–395.
Bergeron, M., Swain, M.S., Reader, T.A., Grondin, L., and Butterworth, R.F. (1990). Effect of ammonia on brain serotonin metabolism in relation to function in the portacaval shunted rat. J. Neurochem. 55:222–229.
Bergqvist, P.B.F., Vogels, B.A.P.M., Bosman, D.K., Maas, M.A.W., Hjorth, S., Chamuleau, R.A.F.M., and Bengtsson, F. (1995). Neocortical dialysate monoamines of rats after acute, subacute, and chronic liver shunt. J. Neurochem. 64:1238–1244.
Bergqvist, P.B.F., Hjorth, S., Apelqvist, G., and Bengtsson, F. (1996a). Acute effects of L-tryptophan on the brain extracellular 5-HT and 5-HIAA levels in chronic experimental portal-systemic encephalopathy. Metab. Brain Dis. 11:269–278.
Bergqvist, P.B.F., Hjorth, S., Audet, R., Apelqvist, G., Bengtsson, F., and Butterworth R.F. (1996b). Ammonium acetate challenge in experimental hepatic encephalopathy induces a transient increase of brain 5-HT release in vivo. Eur. Neuropsychopharmacol. 6:317–324.
Borowsky, B., and Hoffman, B.J. (1995). Neurotransmitter transporters: molecular biology, function, and regulation. Int. Rev. Neurobiol. 38:139–199.
Collard, K.J. (1989). On the mechanism by which extracellular sodium depletion causes 5-hydroxytryptamine release from rat brain synaptosomes. Biochim. Biophys. Acta 984:319–325.
De Deurwaerdére, P., Bonhomme, N., Le Moal, M., and Spampinato, U. (1995). d-Fenfluramine increases striatal extracellular dopamine in vivo independently of serotonergic terminals or dopamine uptake sites. J. Neurochem. 65:1100–1108.
Di Chiara, G. (1990). Brain dialysis of neurotransmitters: a commentary. J. Neurosci. Methods 34:29–34.
Elks, M.L., Youngblood, W.W., and Kizer, J.S. (1979). Serotonin synthesis and release in brain slices: independence of tryptophan. Brain Res. 172:471–486.
Grahame-Smith, D.G. (1971). Studies in vivo on the relationship between brain tryptophan, brain 5-HT synthesis and hyperactivity in rats treated with a monoamine oxidase inhibitor and L-tryptophan. J. Neurochem. 18:1053–1066.
Grahame-Smith, D.G. (1973). Does the total turnover of brain 5-HT reflect the functional activity of 5-HT in brain? In (J. Barchas, and E. Usdin, eds.), Serotonin and Behavior, Academic Press, New York, NY, U.S.A., pp 5–7.
Hjorth, S., and Sharp, T. (1993). In vivo microdialysis evidence for central serotonin1A and serotonin1B autoreceptor blocking properties of the beta adrenoceptor antagonist (-)penbutolol. J. Pharmacol. Exp. Ther. 265:707–712.
Kalén, P., Strecker, R.E., Rosengren, E., and Björklund, A. (1988). Endogenous release of neuronal serotonin and 5-hydroxyindoleacetic acid in the caudate-putamen of rat as revealed by intracerebral dialysis coupled to high-performance liquid chromatography with fluorimetric detection. J. Neurochem. 51:1422–1435.
Lee, S.H., and Fisher, B. (1961). Portacaval shunt in the rat. Surgery 50:668–672.
Mans, A.M., DeJoseph, M.R., Davis, D.W., Viña, J.R. and Hawkins, R.A. (1990). Early establishment of cerebral dysfunction after portacaval shunting. Am. J. Physiol. 259:E104–E110.
Maura, G., Gemignani, A., Versace, P., Martire, M., and Raiteri, M. (1982). Carrier-mediated and carrier-independent release of serotonin from isolated central nerve endings. Neurochem. Int. 4:219–224.
Mousseau, D.D., and Butterworth, R.F. (1994). Current theories on the pathogenesis of hepatic encephalopathy. Exp. Biol. Med. 206:329–344.
Schröder, R., Müller, O., and Bircher, J. (1985). The portacaval and splenocaval shunt in the normal rat. A morphometric and functional reevaluation. J. Hepatol. 1:107–123.
Shields, P.J., and Eccleston, D. (1973). Evidence for the synthesis and storage of 5-hydroxytryptamine in two separate pools in the brain. J. Neurochem. 20:881–888.
Theander, B., Apelqvist, G., Hindfelt, B., Bugge, M., Andersson, G., and Bengtsson, F. (1997). Gender and diurnal effects on specific open-field behavioral patterns in the portacaval shunted rat. Metab. Brain Dis. 12:47–59.
Westerink, B.H.C., Damsma, G., Rollema, H., deVries, J.B., and Horn, A.S. (1987). Scope and limitations of in vivo brain dialysis: a comparison of its application to various neurotransmitter systems. Life Sci. 41:1763–1776.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bergqvist, P.B.F., Hjorth, S., Apelqvist, G. et al. Potassium-Evoked Neuronal Release of Serotonin in Experimental Chronic Portal-Systemic Encephalopathy. Metab Brain Dis 12, 193–202 (1997). https://doi.org/10.1023/B:MEBR.0000007100.04521.6e
Issue Date:
DOI: https://doi.org/10.1023/B:MEBR.0000007100.04521.6e