Abstract
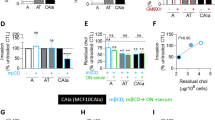
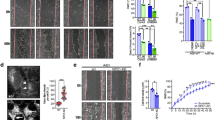
We have investigated the implications of the rise in membrane cholesterol levels on several in vitro and in vivo properties of polyoma virus transformed rat fibroblasts (PyF), with a special emphasis on α5β_1 integrin functions. We show that increased membrane cholesterol causes the PyF cells to change their shape and become more bipolar in appearance. These cells also show significantly higher adhesion to the cell-binding domain of fibronectin, increased localization of α5β1 integrin and talin molecules in focal adhesions and a more robust actin cytoskeleton organization. PyF cells with increased membrane cholesterol show reduced growth in vitro and tumours caused by these cells in nude mice are slow growing. These changes in the growth properties of PyF cells are reversible when the cholesterol levels of PyF cells become normal. Our results suggest that changes in membrane cholesterol levels influence the growth and morphological properties of transformed cells, which can be exploited in controlling the growth of tumours in vivo. (Mol Cell Biochem 265: 83–95, 2004)
Similar content being viewed by others
References
Ruoslahti E: Stretching is good for a cell. Science 276: 1345–1346, 1997
Cos S, Fernandez R, Guezymes A, Sanchez-Barcelo EJ: Influence of melatonin on invasive and metastatic properties of MCF-7 human breast cancer cells. Cancer Res 58: 4383–4390, 1998
Aplin AE, Howe A, Alahari SK, Juliano TI: Signal transduction and signal modulation by cell adhesion receptors. Pharmacol Rev 50(2): 197–263, 1998
Hynes RO: Integrins: bi-directional, allosteric signaling machines. Cell 110(6): 673–687, Sep 20, 2002
Jockush BM, Rudiger M: Cross talk between cell adhesion molecules: Vinculin as a paradigm for regulation by conformation. Trends CellBiol 6: 311–315, 1996
Ben-Ze'ev A: The cytoskeleton of cancer cells. Biochemica et Biophys-ica Acta 780: 197–212, 1985
Ben-Ze'eV: A Cytoskeletal and adhesion proteins as tumor suppressors. Curr Opin Cell Biol 9: 99–108, 1997
Kincha MS, Burridge K: Altered adhesions in ras transformed breast epithelial cells. Biochem Soc Trans 23: 446–458
Gluck U, Kwiatkowski DJ, Ben-Ze'eV A: Suppression of tumourigenic-ity in Simian virus 40-transformed cells transfected with a-actinin cDNA. Proc Natl Acad Sci USA 90: 383–387, 1993
Donald E Ingber: The architecture of life. Sci Am Jan 48–57, 1998
Leibovici J, Klein O, Wollman Y, Donin N, Mahlin T, Shinitzky M: Cell membrane fluidity and Adriamycin retention in a tumor progression.95 model of AKR lymphoma. Biochemica et Biophysica Acta 1281: 182–188, 1996
Podolin DA, Sutherland E, Iwahashi M, Simon FR, Pagliassotti MJ: A high sucrose diet alters the lipid composition and fluidity of liver sinusoidal membranes. Horm-Metab Res 30: 195–199, 1998
Arbogast LY, Rothblat GH, Leslie MH, Cooper RA: Cellular cholesterol ester accumulation induced by free cholesterol rich lipid dispersions. Proc Natl Acad Sci USA 73: 3680–3684, 1976
Hartel S, Diehl HA, Flavio-Ojeda S: Methyl b cyclodextrins and lipo-somes as water soluble carriers for cholesterol incorporation into the membranes and its evaluation by a micro enzymatic fluorescence assay and membrane fluidity sensitive dyes. Anal Biochem 258: 277–284, 1998
Anil Kumar N, Bhattacharya AK, Manogaran PS, Pande G: Modulation of á 5 1 and á v 3 integrins on the cell surface during mitosis. J Cell Biochem 61: 338–349, 1996
Mosmann T: Rapid colorimetric assay for cellular growth and survival. Applications to proliferation and cytotoxicity assays. Immunol Methods 63: 55–63, 1983
Van Blitterswijik WJ, Van Hoeven RP, Vander Meer BW: Lipid struc-tural order parameters (reciprocal of fluidity) in biomembranes derived from steady state fluorescence polarisation measurements. 644: 323–332, 1981
Takagi J, Peter BM, Walz T, Springer TA: Global conformational rear-rangements in integrin extracellular domains in outside-in and inside-out signaling. Cell 110(5): 599–611, Sep 6, 2002
Burridge K, Chrzanowska-Wodnicka M: Focal adhesions, contractility and signaling. Annu Rev Cell Dev Biol 12: 463–519, 1996
Kim M, Carman CV, Springer TA: Bidirectional transmembrane signal-ing by cytoplasmic domain separation in Integrins. Science 301(5640): 1720–1725, Sep 19, 2003
Keely P, Parise L, Juliano R: Integrins and GTPases in tumor cell growth, motility and invasion. Trends Cell Biol 8: 101–106, 1998
Hibbs ML, Xu H, Stacker SA, Springer TA: Regulation of adhesion of ICAM-I by the cytoplasmic domain of LFA-1 integrin Influence of receptor lateral mobility on adhesion strengthening between molecules containing LFA-3 and CD2. J Cell Biol 115: 245–255, 1991
Chan PY, DE, Springer TA: Influence of receptor lateral mobility on adhesion strengthening between molecules containing LFA-3 and CD2. J. Cell Biol 115: 245–255, 1991
Lawrence MB, Dustin ML, Ferguson LM, Golan DE, Springer TA: Influence of receptor lateral mobility on adhesion strengthening be-tween molecules containing LFA-3 and CD2. J Cell Biol 115: 245–255, 1991
Gopalakrishna P, Chaubey SK, Manogaran PS, Pande G: Modu-lation of á 5 1 integrin functions by the phospholipid and choles-terol contents of the membranes. J Cell Biochem 77: 517–528, 2000
Hynes RO: In E. Hay (ed). The Cell Biology of Extracellular Matrix: 2nd edn. Plenum Publishers, New York, pp 295–331, 1981
Freedman VH, Shin S: Cellular tumourigenicity in nude mice: Correla-tion with cell growth in semi-solid medium. Cell 3: 355–359, 1974
Plantefaber LC, Hynes RO: Changes in integrin receptors on oncogeni-cally transformed cells. Cell 56: 281–290, 1989
Gopal Pande: The role of membrane lipids in regulation of integrin functions. Curr Opin Cell Biol 12(5): 569–574, 2000
Gottlieb MH: The reactivity of human erythrocyte membrane choles-terol with cholesterol oxidase. Biochem. Biophys. Acta 466: 422–428, 1977
Yeagle PL: Cholesterol and the cell membrane. Biochem Biophys Acta 822: 267–287, 1985
Gimpl G, Burger K, Fahrenholz F: Cholesterol as modulator of receptor function. Biochemstry 36: 10959–10974, 1997
McIntosh TJ, Magid AD, Simon SA: Cholesterol modifies the short-range repulsive interactions between phosphatidylcholine membranes. Biochemistry 28: 17–25, 1989
Folkman J, Moscona A: The role of cell shape in growth control. Nature 273: 345–349, 1978
Small JV, Rottner K, Kavernia I: Functional design in the actin cy-toskeleton. Curr Opi Cell Biol 11: 54–60, 1999
Schoenwaelder SM, Burrdige K: Bi-directional signaling between cy-toskeleton and integrins. Curr Opi Cell Biol 11: 274–286, 1999
Hall AM: Rho. GTPases and the actin cytoskeleton. Science 279: 509–514, 1998
Mortarini R, Gismondi G, Santoni A, Permiani G, Arichini A: Role of á 5 â 1 receptor in proliferative response of quiescent human melanoma to fibronectin. Cancer Res 52: 4499–4506, 1992
Giancotti FG, Ruoslahti E: Eleveated levels of á 5 â 1 fibronectin receptor suppress the transformed phenotype of Chinese hamster ovary cells. Cell 60: 849–859, 1990
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kaur, T., Gopalakrishna, P., Manogaran, P.S. et al. A correlation between membrane cholesterol level, cell adhesion and tumourigenicity of polyoma virus transformed cells. Mol Cell Biochem 265, 83–95 (2004). https://doi.org/10.1023/B:MCBI.0000044320.65756.14
Issue Date:
DOI: https://doi.org/10.1023/B:MCBI.0000044320.65756.14