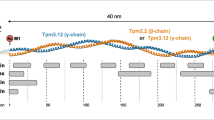
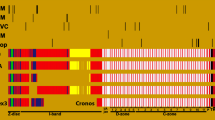
Abstract
Several striated muscle myopathies have been directly linked to mutations in contractile and associated proteins. Troponin T (TnT) is one of the three subunits that form troponin (Tn) which together with tropomyosin is responsible for the regulation of striated muscle contraction. All three subunits of cardiac Tn as well as tropomyosin have been associated with hypertrophic cardiomyopathy (HCM). However, TnT accounts for most of the mutations that cause HCM in these regulatory proteins. To date 30 mutations have been identified in the cardiac TnT (CTnT) gene that results in familial HCM (FHC). The CTnT gene has also been associated with familial dilated cardiomyopathy (DCM). CTnT deficiency is lethal due to impaired cardiac development. A recessive nonsense mutation in the gene encoding slow skeletal TnT has been associated with an unusual, severe form of nemaline myopathy among the Old Order Amish. How each mutation leads to the diverse clinical symptoms associated with FHC, DCM or nemaline myopathy is unclear. However, the use of animal model systems, in particular transgenic mice, has significantly increased our knowledge of normal and myopathic muscle physiology. In this review, we focus on the role of TnT in muscle physiology and disease. (Mol Cell Biochem 263: 115–129, 2004)
Similar content being viewed by others
References
McKenna WJ, Camm AJ: Sudden death in hypertrophic cardiomyopa-thy. Assessment of patients at high risk. Circulation 80: 148 9–1492, 1989
Maron BJ, Gardin JM, Flack JM, Gidding SS, Kurosaki TT, Bild DE: Prevalence of hypertrophic cardiomyopathy in a general population of young adults. Echocardiographic analysis of 4111 subjects in the CAR-DIA Study. Coronary Artery Risk Development in (Young) Adults. Circulation 92: 785–789, 1995
Roberts R: Molecular genetics. Therapy or terror? Circulation 89: 499–502, 1994
Geisterfer-Lowrance AA, Kass S, Tanigawa G, Vosberg HP, McKenna W, Seidman CE, Seidman JG: A molecular basis for familial hyper-trophic cardiomyopathy: A beta cardiac myosin heavy chain gene mis-sense mutation. Cell 62: 999–1006, 1990
Poetter K, Jiang H, Hassanzadeh S, Master SR, Chang A, Dalakas MC, Rayment I, Sellers JR, Fananapazir L, Epstein ND: Mutations in either the essential or regulatory light chains of myosin are associated with a rare myopathy in human heart and skeletal muscle. Nat Genet 13: 63–69, 1996
Thierfelder L, Watkins H, MacRae C, Lamas R, McKenna W, Vosberg HP, Seidman JG, Seidman CE: Alpha-tropomyosin and cardiac troponin T mutations cause familial hypertrophic cardiomyopathy: A disease of the sarcomere. Cell 77: 701–712, 1994
Watkins H, Conner D, Thierfelder L, Jarcho JA, MacRae C, McKenna WJ, Maron BJ, Seidman JG, Seidman CE: Mutations in the cardiac myosin binding protein-C gene on chromosome 11 cause familial hy-pertrophic cardiomyopathy. Nat Genet 11: 434–437, 1995
Bonne G, Carrier L, Bercovici J, Cruaud C, Richard P, Hainque B, Gautel M, Labeit S, James M, Beckmann J, Weissenbach J, Vosberg HP, Fiszman M, Kamajda M, Schwartz K: Cardiac myosin binding protein-C gene splice acceptor site mutation is associated with familial hypertrophic cardiomyopathy. Nat Genet 11: 438–440, 1995
Kimura A, Harada H, Park JE, Nishi H, Satoh M, Takahashi M, Hiroi S, Sasaoka T, Ohbuchi N, Nakamura T, Koyanagi T, Hwang TH, Choo JA, Chung KS, Hasegawa A, Nagai R, Okazaki O, Nakamura H, Matsuzaki M, Sakamoto T, Toshima H, Koga Y, Imaizumi T, Sasazuki T: Mutations in the cardiac troponin I gene associated with hypertrophic cardiomyopathy. Nat Genet 16:379–382, 1997
Mogensen J, Klausen IC, Pedersen AK, Egeblad H, Bross P, Kruse TA, Gregersen N, Hansen PS, Baandrup U, Borglum AD: Alpha-cardiac actin is a novel disease gene in familial hypertrophic cardiomyopathy. J Clin Invest 103: R39–R43, 1999
Satoh M, Takahashi M, Sakamoto T, Hiroe M, Marumo F, Kimura A: Structural analysis of the titin gene in hypertrophic cardiomyopathy: Identification of a novel disease gene. Biochem Biophys Res Commun 262: 411–417, 1999
Hoffmann B, Schmidt-Traub H, Perrot A, Osterziel KJ, Gessner R: First mutation in cardiac troponin C, L29Q, in a patient with hyper-trophic cardiomyopathy. Hum Mutat 17: 524, 2001
Grunig E, Tasman JA, Kucherer H, Franz W, Kubler W, Katus HA: Frequency and phenotypes of familial dilated cardiomyopathy. J Am Coll Cardiol 31: 186–194, 1998
Kamisago M, Sharma SD, DePalma SR, Solomon S, Sharma P, McDonough B, Smoot L, Mullen MP, Woolf PK, Wigle ED, Seidman JG, Seidman CE: Mutations in sarcomere protein genes as a cause of dilated cardiomyopathy. N Engl J Med 343: 1688–1696, 2000
Li D, Czernuszewicz GZ, Gonzalez O, Tapscott T, Karibe A, Durand JB, Brugada R, Hill R, Gregoritch JM, Anderson JL, Quinones M, Bachinski LL, Roberts R: Novel cardiac troponin T mutation as a cause of familial dilated cardiomyopathy. Circulation 104: 2188–2193, 2001
Ilkovski B, Cooper ST, Nowak K, Ryan MM, Yang N, Schnell C, Durling HJ, Roddick LG, Wilkinson I, Kornberg AJ, Collins KJ, Wallace G, Gunning P, Hardeman EC, Laing NG, North KN: Nemaline myopathy caused by mutations in the muscle alpha-skeletal-actin gene. Am J Hum Genet 68: 1333–1343, 2001
Durling HJ, Reilich P, Muller-Hocker J, Mendel B, Pongratz D, Wallgren-Pettersson C, Gunning P, Lochmuller H, Laing NG: De novo missense mutation in a constitutively expressed exon of the slowalpha-tropomyosin gene TPM3 associated with an atypical, sporadic case of nemaline myopathy. Neuromuscul Disord 12:947–951, 2002
Donner K, Ollikainen M, Ridanpaa M, Christen HJ, Goebel HH, de Visser M, Pelin K, Wallgren-Pettersson C: Mutations in the beta-tropomyosin (TPM2) gene—A rare cause of nemaline myopathy. Neuromuscul Disord 12: 151–158, 2002
Wallgren-Pettersson C, Donner K, Sewry C, Bijlsma E, Lammens M, Bushby K, Giovannucci Uzielli ML, Lapi E, Odent S, Akcoren Z, Topaloglu H, Pelin K: Mutations in the nebulin gene can cause se-vere congenital nemaline myopathy. Neuromuscul Disord 12: 674–679, 2002
Johnston JJ, Kelley RI, Crawford TO, Morton DH, Agarwala R, Koch T, Schaffer AA, Francomano CA, Biesecker LG: A novel nemaline myopathy in the Amish caused by a mutation in troponin T1. Am J Hum Genet 67: 814–821, 2000
Sanoudou D, Beggs AH: Clinical and genetic heterogeneity in nema-line myopathy—A disease of skeletal muscle thin filaments. Trends Mol Med 7: 362–368, 2001
Ebashi S: Regulation of the myosin-actin interaction by the Ca 2 +-troponin–tropomyosin system. J Biochem (Tokyo) 79: 48P–49P, 1976
Zot AS, Potter JD: Structural aspects of troponin-tropomyosin regula-tion of skeletal muscle contraction. Annu Rev Biophys Biophys Chem 16: 535–559, 1987
White SP, Cohen C, Phillips GN Jr.: Structure of co-crystals of tropomyosin and troponin. Nature 325: 826–828, 1987
Stefancsik R, Jha PK, Sarkar S: Identification and mutagenesis of a highly conserved domain in troponin T responsible for troponin I bind-ing: Potential role for coiled coil interaction. Proc Natl Acad Sci USA 95: 957–962, 1998
Takeda S, Yamashita A, Maeda K, Maeda Y: Structure of the core domain of human cardiac troponin in the Ca 2 + saturated form. Nature 424: 35–41, 2003
Potter JD, Sheng Z, Pan BS, Zhao J: A direct regulatory role for tro-ponin T and a dual role for troponin C in the Ca 2 + regulation of muscle contraction. J Biol Chem 270: 2557–2562, 1995
Fraser ID, Marston SB: In vitro motility analysis of actin-tropomyosin regulation by troponin and calcium. The thin filament is switched as a single cooperative unit. J Biol Chem 270: 7836–7841, 1995
Sehnert AJ, Huq A, Weinstein BM, Walker C, Fishman M, Stainier DY: Cardiac troponin T is essential in sarcomere assembly and cardiac contractility. Nat Genet 31: 106–110, 2002
Perry SV: Troponin T: genetics, properties and function. J Muscle Res Cell Motil 19: 575–602, 1998
Farza H, Townsend PJ, Carrier L, Barton PJ, Mesnard L, Bahrend E, Forissier JF, Fiszman M, Yacoub MH, Schwartz K: Genomic organi-sation, alternative splicing and polymorphisms of the human cardiac troponin T gene. J Mol Cell Cardiol 30: 1247–1253, 1998
Townsend PJ, Barton PJ, Yacoub MH, Farza H: Molecular cloning of human cardiac troponin T isoforms: Expression in developing and failing heart. J Mol Cell Cardiol 27: 2223–2236, 1995
Antin PB, Zhang W, Bales MA, Garriock RJ, Yatskievych TA: Pre-cocious expression of cardiac troponin T in early chick embryos is independent of bone morphogenetic protein signaling. Dev Dyn 225: 376, 2002
Gomes AV, Guzman G, Zhao J, Potter JD: Cardiac troponin T isoforms affect the Ca 2 + sensitivity and inhibition of force development. Insights into the role of troponin T isoforms in the heart. J Biol Chem 277: 35341–35349, 2002
Schachat FH, Diamond MS, Brandt PW: Effect of different troponin T-tropomyosin combinations on thin filament activation. J Mol Biol 198: 551–554, 1987
Tobacman LS, Lee R: Isolation and functional comparison of bovine cardiac troponin T isoforms. J Biol Chem 262: 4059–4064, 1987
Knollmann BC, Potter JD: Altered regulation of cardiac muscle con-traction by troponin T mutations that cause familial hypertrophic car-diomyopathy. Trends Cardiovasc Med 11: 206–212, 2001
Watkins H, McKenna WJ, Thierfelder L, Suk HJ, Anan R, O'Donoghue A, Spirito P, Matsumori A, Moravec CS, Seidman JG, Seidman CE: Mutations in the genes for cardiac troponin T and alpha-tropomyosin in hypertrophic cardiomyopathy. N Engl J Med 332: 1058–1064, 1995
Anan R, Shono H, Kisanuki A, Arima S, Nakao S, Tanaka H: Patients with familial hypertrophic cardiomyopathy caused by a Phe110Ile mis-sense mutation in the cardiac troponin T gene have variable cardiac morphologies and a favorable prognosis. Circulation 98: 391–397, 1998
Forissier JF, Carrier L, Farza H, Bonne G, Bercovici J, Richard P, Hainque B, Townsend PJ, Yacoub MH, Faure S, Dubourg O, Millaire A, Hagege AA, Desnos M, Komajda M, Schwartz K: Codon 102 of the cardiac troponin T gene is a putative hot spot for mutations in familial hypertrophic cardiomyopathy. Circulation 94: 3069–3073, 1996
Lin T, Ichihara S, Yamada Y, Nagasaka T, Ishihara H, Nakashima N, Yokota M: Phenotypic variation of familial hypertrophic cardiomy-opathy caused by the Phe(110) → Ile mutation in cardiac troponin T. Cardiology 93: 155–162, 2000
Ho CY, Lever HM, DeSanctis R, Farver CF, Seidman JG, Seidman CE: Homozygous mutation in cardiac troponin T: Implications for hypertrophic cardiomyopathy. Circulation 102: 1950–1955, 2000
Nakajima-Taniguchi C, Matsui H, Fujio Y, Nagata S, Kishimoto T, Yamauchi-Takihara K: Novel missense mutation in cardiac troponin T gene found in Japanese patient with hypertrophic cardiomyopathy. J Mol Cell Cardiol 29: 839–843, 1997
Varnava A, Baboonian C, Davison F, de Cruz L, Elliott PM, Davies MJ, McKenna WJ: A new mutation of the cardiac troponin T gene causing familial hypertrophic cardiomyopathy without left ventricular hypertrophy. Heart 82: 621–624, 1999
Szczesna D, Zhang R, Zhao J, Jones M, Guzman G, Potter JD: Altered regulation of cardiac muscle contraction by troponin T mutations that cause familial hypertrophic cardiomyopathy. J Biol Chem 275: 624–630, 2000
Tobacman LS, Lin D, Butters C, Landis C, Back N, Pavlov D, Homsher E: Functional consequences of troponin T mutations found in hypertrophic cardiomyopathy. J Biol Chem 274: 28363–28370, 1999
Nakaura H, Yanaga F, Ohtsuki I, Morimoto S: Effects of missense mutations Phe110Ile and Glu244Asp in human cardiac troponin T on force generation in skinned cardiac muscle fibers. J Biochem (Tokyo) 126: 457–460, 1999
Takahashi-Yanaga F, Ohtsuki I, Morimoto S: Effects of troponin T mu-tations in familial hypertrophic cardiomyopathy on regulatory func-tions of other troponin subunits. J Biochem (Tokyo) 130: 127–131, 2001
Harada K, Takahashi-Yanaga F, Minakami R, Morimoto S, Ohtsuki I: Functional consequences of the deletion mutation deltaGlu160 in human cardiac troponin T. J Biochem (Tokyo) 127: 263–268, 2000
Mukherjea P, Tong L, Seidman JG, Seidman CE, Hitchcock-DeGregori SE: Altered regulatory function of two familial hypertrophic cardiomy-opathy troponin T mutants. Biochemistry 38: 13296–13301, 1999
Nakaura H, Morimoto S, Yanaga F, Nakata M, Nishi H, Imaizumi T, Ohtsuki I: Functional changes in troponin T by a splice donor site mutation that causes hypertrophic cardiomyopathy. Am J Physiol 277: C225–C232, 1999
Redwood C, Lohmann K, Bing W, Esposito GM, Elliott K, Abdul-razzak H, Knott A, Purcell I, Marston S, Watkins H: Investigation of a truncated cardiac troponin T that causes familial hypertrophic cardiomyopathy: Ca 2 + regulatory properties of reconstituted thin fila-ments depend on the ratio of mutant to wild-type protein. Circ Res 86: 1146–1152, 2000
Schonberger J, Seidman CE: Many roads lead to a broken heart: The genetics of dilated cardiomyopathy. Am J Hum Genet 69: 249–260, 2001
Hanson EL, Jakobs PM, Keegan H, Coates K, Bousman S, Dienel NH, Litt M, Hershberger RE: Cardiac troponin T lysine 210 deletion in a family with dilated cardiomyopathy. J Card Fail 8: 28–32, 2002
Moolman JC, Corfield VA, Posen B, Ngumbela K, Seidman C, Brink PA, Watkins H: Sudden death due to troponin T mutations. J Am Coll Cardiol 29: 549–555, 1997
Fujino N, Shimizu M, Ino H, Okeie K, Yamaguchi M, Yasuda T, Kokado H, Mabuchi H: Cardiac troponin T Arg92Trp mutation and progression from hypertrophic to dilated cardiomyopathy. Clin Cardiol 24: 397–402, 2001
Fujino N, Shimizu M, Ino H, Yamaguchi M, Yasuda T, Nagata M, Konno T, Mabuchi H: A novel mutation Lys273Glu in the cardiac troponin T gene shows high degree of penetrance and transition from hypertrophic to dilated cardiomyopathy. Am J Cardiol 89: 29–33, 2002
Robinson P, Mirza M, Knott A, Abdulrazzak H, Willott R, Marston S, Watkins H, Redwood C: Alterations in thin filament regulation induced by a human cardiac troponin T mutant that causes dilated cardiomy-opathy are distinct from those induced by troponin T mutants that cause hypertrophic cardiomyopathy. J Biol Chem 277: 40710–40716, 2002
Morimoto S, Lu QW, Harada K, Takahashi-Yanaga F, Minakami R, Ohta M, Sasaguri T, Ohtsuki I: Ca(2 +)-desensitizing effect of a deletion mutation Delta K210 in cardiac troponin T that causes familial dilated cardiomyopathy. Proc Natl Acad Sci USA 99: 913–918, 2002
Marian AJ, Roberts R: On Koch's postulates, causality and genetics of cardiomyopathies. J Mol Cell Cardiol 34: 971–974, 2002
Oberst L, Zhao G, Park JT, Brugada R, Michael LH, Entman ML, Roberts R, Marian AJ: Dominant-negative effect of a mutant cardiac troponin T on cardiac structure and function in transgenic mice. J Clin Invest 102: 1498–1505, 1998
Lim DS, Oberst L, McCluggage M, Youker K, Lacy J, DeMayo F, Entman ML, Roberts R, Michael LH, Marian AJ: Decreased left ven-tricular ejection fraction in transgenic mice expressing mutant cardiac troponin T-Q(92), responsible for human hypertrophic cardiomyopa-thy. J Mol Cell Cardiol 32: 365–374, 2000
Tardiff JC, Hewett TE, Palmer BM, Olsson C, Factor SM, Moore RL, Robbins J, Leinwand LA: Cardiac troponin T mutations result in allele-specific phenotypes in a mouse model for hypertrophic car-diomyopathy. J Clin Invest 104: 469–481, 1999
Tardiff JC, Factor SM, Tompkins BD, Hewett TE, Palmer BM, Moore RL, Schwartz S, Robbins J, Leinwand LA: Atruncated cardiac troponin T molecule in transgenic mice suggests multiple cellular mechanisms for familial hypertrophic cardiomyopathy. J Clin Invest 101: 2800–2811, 1998
Frey N, Franz WM, Gloeckner K, Degenhardt M, Muller M, Muller O, Merz H, Katus HA: Transgenic rat hearts expressing a human car-diac troponin T deletion reveal diastolic dysfunction and ventricular arrhythmias. Cardiovasc Res 47: 254–264, 2000
Knollmann BC, Blatt SA, Horton K, de Freitas F, Miller T, Bell M, Housmans PR, Weissman NJ, Morad M, Potter JD: Inotropic stim-ulation induces cardiac dysfunction in transgenic mice expressing a troponin T (I79N) mutation linked to familial hypertrophic cardiomy-opathy. J Biol Chem 276: 10039–10048, 2001
Miller T, Szczesna D, Housmans PR, Zhao J, de Freitas F, Gomes AV, Culbreath L, McCue J, Wang Y, Xu Y, Kerrick WG, Potter JD: Abnormal contractile function in transgenic mice expressing a familial hypertrophic cardiomyopathy-linked troponin T (I79N) mutation. J Biol Chem 276: 3743–3755, 2001
Gomes AV, Miller T, Jaquez O, Ampie L, Potter JD: Impaired regu-latory properties of a truncated slow skeletal troponin T that causes nemaline myopathy. Biophys J 84: 319A, 2003
Yuasa K, Michibata H, Omori K, Yanaka N: A novel interaction of cGMP-dependent protein kinase I with troponin T. J Biol Chem 274: 37429–37434, 1999
Jaquet K, Fukunaga K, Miyamoto E, Meyer HE: A site phosphory-lated in bovine cardiac troponin T by cardiac CaM kinase II. Biochim Biophys Acta 1248: 193–195, 1995
Noland TA Jr., Kuo JF: Protein kinase C phosphorylation of cardiac troponin I and troponin T inhibits Ca(2 +)-stimulated MgATPase activ-ity in reconstituted actomyosin and isolated myofibrils, and decreases actin-myosin interactions. J Mol Cell Cardiol 25: 53–65, 1993
Morimoto S, Yanaga F, Minakami R, Ohtsuki I: Ca 2 +-sensitizing ef-fects of the mutations at Ile-79 and Arg-92 of troponin Tin hypertrophic cardiomyopathy. Am J Physiol 275: C200–C207, 1998
Biesiadecki BJ, Elder BD, Yu ZB, Jin JP: Cardiac troponin T variants produced by aberrant splicing of multiple exons in animals with high instances of dilated cardiomyopathy. J Biol Chem 107, 2002
Palm T, Graboski S, Hitchcock-DeGregori SE, Greenfield NJ: Disease-causing mutations in cardiac troponin T: Identification of a critical tropomyosin-binding region. Biophys J 81: 2827–2837, 2001
Sarko J, Pollack CV Jr.: Cardiac troponins. J Emerg Med 23: 57–65, 2002
Mair J: Cardiac troponin I and troponin T: Are enzymes still relevant as cardiac markers? Clin Chim Acta 257: 99–115, 1997
Wu AH, Feng YJ, Moore R, Apple FS, McPherson PH, Buechler KF, Bodor G: Characterization of cardiac troponin subunit release into serum after acute myocardial infarction and comparison of assays for troponin T and I. American Association for Clinical Chemistry Sub-committee on cTnI Standardization. Clin Chem 44: 1198–1208, 1998
Panteghini M, Gerhardt W, Apple FS, Dati F, Ravkilde J, Wu AH: Quality specifications for cardiac troponin assays. Clin Chem Lab Med 39: 175–179, 2001
Katus HA, Remppis A, Scheffold T, Diederich KW, Kuebler W: Intra-cellular compartmentation of cardiac troponin T and its release kinetics in patients with reperfused and nonreperfused myocardial infarction. Am J Cardiol 67: 1360–1367, 1991
Voss EM, Sharkey SW, Gernert AE, Murakami MM, Johnston RB, Hsieh CC, Apple FS: Human and canine cardiac troponin T and crea-tine kinase-MB distribution in normal and diseased myocardium. In-farct sizing using serum profiles. Arch Pathol Lab Med 119: 799–806, 1995
Ricchiuti V, Zhang J, Apple FS: Cardiac troponin I and T alterations in hearts with severe left ventricular remodeling. Clin Chem 43: 990–995, 1997
Varnava AM, Elliott PM, Baboonian C, Davison F, Davies MJ, McKenna WJ: Hypertrophic cardiomyopathy: Histopathological fea-tures of sudden death in cardiac troponin T disease. Circulation 104:1380–1384, 2001
Marian AJ, Roberts R: The molecular genetic basis for hypertrophic cardiomyopathy. J Mol Cell Cardiol 33:655–670, 2001
D'Cruz LG, Baboonian C, Phillimore HE, Taylor R, Elliott PM, Varnava A, Davison F, McKenna WJ, Carter ND: Cytosine methylation confers instability on the cardiac troponin T gene in hypertrophic car-diomyopathy. J Med Genet 37: E18, 2000
Ackerman MJ, VanDriest SL, Ommen SR, Will ML, Nishimura RA, Tajik AJ, Gersh BJ: Prevalence and age-dependence of malignant mu-tations in the beta-myosin heavy chain and troponin T genes in hy-pertrophic cardiomyopathy: A comprehensive outpatient perspective. J AmColl Cardiol 39: 2042–2048, 2002
Yanaga F, Morimoto S, Ohtsuki I: Ca 2 + sensitization and potentiation of the maximum level of myofibrillar ATPase activity caused by mu-tations of troponin T found in familial hypertrophic cardiomyopathy. J Biol Chem 274: 8806–8812, 1999
Morimoto S, Nakaura H, Yanaga F, Ohtsuki I: Functional consequences of a carboxyl terminal missense mutation Arg278Cys in human cardiac troponin T. Biochem Biophys Res Commun 261: 79–82, 1999
Lin D, Bobkova A, Homsher E, Tobacman LS: Altered cardiac troponin T in vitro function in the presence of a mutation implicated in familial hypertrophic cardiomyopathy. J Clin Invest 97: 2842–2848, 1996
Harada K, Szczesna D, Hernandez O, Potter JD: Effect of human cardiac troponin T mutations linked to familial hypertrophic cardiomy-opathy (FHC) on the force-pCa relationship in skinned human muscle fibers. Biophys J 82: A391, 2002
Sweeney HL, Feng HS, Yang Z, Watkins H: Functional analyses of troponin T mutations that cause hypertrophic cardiomyopathy: insights into disease pathogenesis and troponin function. Proc Natl Acad Sci USA 95: 14406–14410, 1998
Watkins H, Seidman CE, Seidman JG, Feng HS, Sweeney HL: Expression and functional assessment of a truncated cardiac troponin T that causes hypertrophic cardiomyopathy. Evidence for a dominant negative action. J Clin Invest 98: 2456–2461, 1996
Rust EM, Albayya FP, Metzger JM: Identification of a con-tractile deficit in adult cardiac myocytes expressing hypertrophic cardiomyopathy-associated mutant troponin T proteins. J Clin Invest 103: 1459–1467, 1999
Koga Y, Toshima H, Kimura A, Harada H, Koyanagi T, Nishi H, Nakata M, Imaizumi T: Clinical manifestations of hypertrophic cardiomyopa-thy with mutations in the cardiac beta-myosin heavy chain gene or cardiac troponin T gene. J Card Fail 2: S97–S103, 1996
Elliott PM, D'Cruz L, McKenna WJ: Late-onset hypertrophic car-diomyopathy caused by a mutation in the cardiac troponin T gene [letter]. N Engl J Med 341: 1855–1856, 1999
Erdmann FJ, Wischke S, Riedel K, Kallisch H, Fleck ME, Regitz-Zagrosek FV: A new mutation (Arg-278-Pro) in the cardiac troponin T gene (TNNT2) was identified in one patient with typical hypertrophic cardiomyopathy (HCM). Circulation 98: 1273, 1998
Watkins H, MacRae C, Thierfelder L, Chou YH, Frenneaux M, McKenna W, Seidman JG, Seidman CE: A disease locus for familial.129 hypertrophic cardiomyopathy maps to chromosome 1q3. Nat Genet 3: 333–337, 1993
Richard P, Charron P, Carrier L, Ledeuil C, Cheav T, Pichereau C, Benaiche A, Isnard R, Dubourg O, Burban M, Gueffet JP, Millaire A, Desnos M, Schwartz K, Hainque B, Komajda M. Hypertrophic Cardiomyopathy. Distribution of disease genes, spectrum of mutations, and implications for a molecular diagnosis strategy. Circulation 107: 2227–2232, 2003
Van Driest SL, Ellsworth EG, Ommen SR, Tajik AJ, Gersh BJ, Ackerman MJ. Prevalence and spectrum of thin filament mutations in an outpatient referral population with hypertrophic cardiomyopathy. Circulation 108: 445–451, 2003
Garcia-Castro M, Reguero JR, Batalla A, Diaz-Molina B, Gonzalez P, Alvarez V, Cortina A, Cubero GI, Coto E. Hypertrophic cardiomy-opathy: Low frequency of mutations in the beta-myosin heavy chain (MYH7) and cardiac troponin T (TNNT2) genes among Spanish pa-tients. Clin Chem 49: 1279–1285, 2003
Venkatraman G, Harada K, Gomes AV, Kerrick GW, Potter JD. Different functional properties of Troponin T mutants that cause dilated cardiomyopathy. J Biol Chem 278: 41670–41676, 2003.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Gomes, A.V., Barnes, J.A., Harada, K. et al. Role of troponin T in disease. Mol Cell Biochem 263, 115–129 (2004). https://doi.org/10.1023/B:MCBI.0000041853.20588.a0
Issue Date:
DOI: https://doi.org/10.1023/B:MCBI.0000041853.20588.a0