Abstract
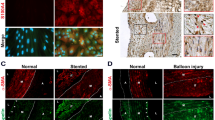
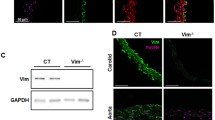
In search of early structural markers of arteriogenesis, we studied the expression of gap junction proteins as well as of contractile and cytoskeletal proteins in smooth muscle cells (SMCs) during coronary collateral vessel growth induced by chronic occlusion of the left circumflex artery (LCx) in the dog heart. We used confocal microscopy with antibodies against connexin37 (Cx37), α-smooth muscle actin (α-SM actin), calponin, desmin and vinculin. The quantitative confocal analysis of immunofluorescence intensity showed that (1) in normal vessels (NV), Cx37 was present in endothelium only, not in SMC. Calponin, α-SM actin, desmin and vinculin were evenly expressed in SMC. (2) In early growing V (EV) with minimal intima formation, α-SM actin, calponin and vinculin showed little change in SMC, but desmin was 3.3 times lower than in NV, and Cx37 was induced (NV 0 arbitrary units/μm2, EV 50.3). (3) In actively growing V (AV), α-SM actin, calponin and vinculin were 3− , 3.3− and 2.9-fold lower, respectively, in the neointima as compared to the media. However, Cx37 was 48.2 AU/μm2 in the media and 15.8 AU/μm2in the neointima. Desmin was almost absent in the neointima and 5-fold reduced in the media. SMC, strongly positive for α-SM actin and calponin, expressed Cx37. Our findings indicate that induction of Cx37 and reduction of desmin precede the phenotypic changes of SMCs, which are characterized by down-regulation of α-SM actin, calponin and vinculin, and the formation of a neointima. An altered expression of Cx37 and desmin, therefore, are early markers for arteriogenesis in dog heart. (Mol Cell Biochem 262: 17–23, 2004)
Similar content being viewed by others
References
Mosse PR, Campbell GR, Campbell JH: Smooth muscle phenotypic expression in human carotid arteries. II. Atherosclerosis-free diffuse intimal thickenings compared with the media. Arteriosclerosis 6(suppl 6): 664–649, 1986
Giuriato L, Scatena M, Chiavegato A, Zanellato AM, Guidolin D, Pauletto P, Sartore S: Localization and smooth muscle cell composition of atherosclerotic lesions inWatanabe heritable hyperlipidemic rabbits. Arterioscler Thromb 13(suppl 3): 347–359, 1993
Kocher O, Gabbiani G: Cytoskeletal features of normal and atheromatous human arterial smooth muscle cells. Hum Pathol 17(suppl 9): 875–880, 1986
Kocher O, Gabbiani F, Gabbiani G, Reidy MA, Cokay MS, Peters H, Huttner I: Phenotypic features of smooth muscle cells during the evolution of experimental carotid artery intimal thickening. Biochemical and morphologic studies. Lab Invest 65(suppl 4): 459–470, 1991
Glukhova MA, Kabakov AE, Frid MG, Ornatsky OI, Belkin AM, Mukhin DN, Orekhov AN, Koteliansky VE, Smirnov VN: Modulation of human aorta smooth muscle cell phenotype: A study of musclespecific variants of vinculin, caldesmon, and actin expression. Proc Natl Acad Sci USA 85(suppl 24): 9542–9546, 1988
Owens GK: Regulation of differentiation of vascular smooth muscle cells. Physiol Rev 75(suppl 3): 487–517, 1995
Wolf C, Cai WJ, Vosschulte R, Koltai S, Mousavipour D, Scholz D, Afsah-Hedjri A, Schaper W, Schaper J: Vascular remodeling and altered protein expression during growth of coronary collateral arteries. J Mol Cell Cardiol 30(suppl 11): 2291–2305, 1998
Cai W, Vosschulte R, Afsah-Hedjri A, Koltai S, Kocsis E, Scholz D, Kostin S, Schaper W, Schaper J: Altered balance between extracellular proteolysis and antiproteolysis is associated with adaptive coronary arteriogenesis. J Mol Cell Cardiol 32(suppl 6): 997–1011, 2000
Cai WJ, Koltai S, Kocsis E, Scholz D, Kostin S, Luo X, Schaper W, Schaper J: Remodeling of the adventitia during coronary arteriogenesis. Am J Physiol Heart Circ Physiol 284: H31–H40, 2003
Schaper W: The Collateral Circulation of the Heart. Elsevier /North-Holland, Amsterdam/London, 1971
Schaper J, Borgers M, Schaper W: Ultrastructure of ischemia-induced changes in the precapillary anastomotic network of the heart. Am J Cardiol 29: 851–859, 1972
Schaper W: Collateral Circulation. V. Mechanisms of collateral enlargement. In: W. Schaper (ed.). The Pathophysiology of Myocardial Perfusion Elsevier /North-Holland/Biomedical Press Amsterdam/New York/Oxford pp. 458–470, 1979
Li Z, Marchand P, Humbert J, Babinet C, Paulin D: Desmin sequence elements regulating skeletal muscle-specific expression in transgenic mice. Development 117(suppl 2): 947–959, 1993
Agbulut O, Li Z, Perie S, Ludosky MA, Paulin D, Cartaud J, Butler-Browne G: Lack of desmin results in abortive muscle regeneration and modifications in synaptic structure. Cell Motil Cytoskeleton 49 (suppl 2): 51–66, 2001
Li Z, Colucci-Guyon E, Pincon-Raymond M, Mericskay M, Pournin S, Paulin D, Babinet C: Cardiovascular lesions and skeletal myopathy in mice lacking desmin. Dev Biol 175(2): 362–366, 1996
Li Z, Mericskay M, Agbulut O, Butler-Browne G, Carlsson L, Thornell L-E, Babinet C, Paulin D: Desmin is essential for the tensile strength and integrity of myofibrils but not for myogenic commitment, differentiation, and fusion of skeletal muscle. J Cell Biol 139(suppl 1): 129–144, 1997
de Wit C, Roos F, Bolz SS, Kirchhoff S, Kruger O, Willecke K, Pohl U: Impaired conduction of vasodilation along arterioles in connexin40-deficient mice. Circ Res 86(suppl 6): 649–655, 2000
Christ GJ, Spray DC, el-Sabban M, Moore LK, Brink PR: Gap junctions in vascular tissues. Evaluating the role of intercellular communication in the modulation of vasomotor tone. Circ Res 79(suppl 4): 631–646, 1996
Blackburn JP, Peters NS, Yeh HI, Rothery S, Green CR, Severs NJ: Upregulation of connexin43 gap junctions during early stages of human coronary atherosclerosis. Arterioscler Thromb Vasc Biol 15(suppl 8): 1219–1228, 1995
Yeh HI, Lupu F, Dupont E, Severs NJ: Upregulation of connexin43 gap junctions between smooth muscle cells after balloon catheter injury in the rat carotid artery. Arterioscler Thromb Vasc Biol 17(suppl 11): 3174–1384, 1997
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Cai, WJ., Kocsis, E., Scholz, D. et al. Presence of Cx37 and lack of desmin in smooth muscle cells are early markers for arteriogenesis. Mol Cell Biochem 262, 17–23 (2004). https://doi.org/10.1023/B:MCBI.0000038201.43148.20
Issue Date:
DOI: https://doi.org/10.1023/B:MCBI.0000038201.43148.20