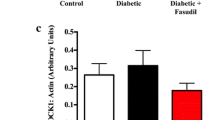
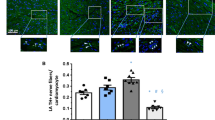
Abstract
Previous studies have shown that the renin–angiotensin system (RAS) is activated in diabetes and this may contribute to the subcellular remodelling and heart dysfunction in this disease. Therefore, we examined the effects of RAS blockade by enalapril, an angiotensin-converting enzyme inhibitor, and losartan, an angiotensin receptor AT1 antagonist, on cardiac function, myofibrillar and myosin ATPase activity as well as myosin heavy chain (MHC) isozyme expression in diabetic hearts. Diabetes was induced in rats by a single injection of streptozotocin (65 mg/kg; i.v.) and these animals were treated with and without enalapril (10 mg/kg/day; oral) or losartan (20 mg/kg/day; oral) for 8 weeks. Enalapril or losartan prevented the depressions in left ventricular rate of pressure development, rate of pressure decay and ventricular weight seen in diabetic animals. Both drugs also attenuated the decrease in myofibrillar Ca2+-ATPase, Mg2+-ATPase and myosin ATPase activity seen in diabetic rats. The diabetes-induced increase in β-MHC content and gene expression as well as the decrease in α-MHC content and mRNA levels were also prevented by enalapril and losartan. These results suggest the occurrence of myofibrillar remodelling in diabetic cardiomyopathy and provide evidence that the beneficial effects of RAS blockade in diabetes may be associated with attenuation of myofibrillar remodelling in the heart. (Mol Cell Biochem 261: 271–278, 2004)
Similar content being viewed by others
References
Fein FS, Malhotra A, Miller-Green B, Scheuer J, Sonnenblick EH: Diabetic cardiomyopathy in rats: Mechanical and biochemical response to different insulin doses. Am J Physiol 247: H817–H823, 1984
Malhotra A, Lopez MC, Nakouzi A: Troponin subunits contribute to altered myosin ATPase activity in diabetic cardiomyopathy. Mol Cell Biochem 151: 165–172, 1995
Fein FS, Strobeck JE, Malhotra A, Scheuer J, Sonnenblick EH: Reversibility of diabetic cardiomyopathy with insulin in rats. Circ Res 49: 1251–1261, 1981
Pierce GN, Dhalla NS: Cardiac myofibrillar ATPase activity in diabetic rats. J Mol Cell Cardiol 13: 1063–1069, 1981
Takeda N, Dixon IM, Hata T, Elimban V, Shah KR, Dhalla NS: Sequence of alterations in subcellular organelles during the development of heart dysfunction in diabetes. Diabetes Res Clin Pract 30: 113–122, 1996
Vadlamudi RV, Rodgers RL, McNeill JH: The effect of chronic alloxan-and streptozotocin-induced diabetes on isolated rat heart performance. Can J Physiol Pharmacol 60: 902–911, 1982
Dhalla NS, Pierce GN, Innes IR, Beamish RE: Pathogenesis of cardiac dysfunction in diabetes mellitus. Can J Cardiol 1: 263–281, 1985
Dillmann WH: Diabetes mellitus induces changes in cardiac myosin of the rat. Diabetes 29: 579–582, 1980
Liu X, Takeda N, Dhalla NS: Troponin I phosphorylation in heart homogenate from diabetic rat. Biochim Biophys Acta 1316: 78–84, 1996
Malhotra A, Sanghi V: Regulation of contractile proteins in diabetic heart. Cardiovasc Res 34: 34–40, 1997
Pierce GN, Dhalla NS: Mechanisms of the defect in cardiac myofibrillar function during diabetes. Am J Physiol 248: E170–E175, 1985
Dhalla NS, Liu X, Panagia V, Takeda N: Subcellular remodelling and heart dysfunction in chronic diabetes. Cardiovasc Res 40: 239–247, 1998
Eisenberg E, Greene LE: The relation of muscle biochemistry to muscle physiology. Annu Rev Physiol 42: 293–309, 1980
Cassis LA: Downregulation of the renin-angiotensin system in streptozotocin-diabetic rats. Am J Physiol 262: E105–E109, 1992
Khatter JC, Sadri P, Zhang M, Hoeschen RJ: Myocardial angiotensin II (Ang II) receptors in diabetic rats. Ann N Y Acad Sci 793: 466–472, 1996
Rett K, Jauch KW, Wicklmayr M, Dietze G, Fink E, Mehnert H: Angiotensin-converting enzyme inhibitors in diabetes: Experimental and human experience. Postgrad Med J 62: 59–64, 1986
Sechi LA, Griffin CA, Schambelan M: The cardiac renin-angiotensin system in STZ-induced diabetes. Diabetes 43: 1180–1184, 1994
Hoenack C, Rosen P: Inhibition of angiotensin type 1 receptor prevents decline of glucose transporter (GLUT4) in diabetic rat heart. Diabetes 45: S82–S87, 1996
Rosen P, Rump AF, Rosen R: Influence of angiotensin-converting enzyme inhibition by fosinopril on myocardial perfusion in streptozotocin-diabetic rats. J Cardiovasc Pharmacol 27: 64–70, 1996
Rosen R, Rump AF, Rosen P: The ACE-inhibitor captopril improves myocardial perfusion in spontaneously diabetic (BB) rats. Diabetologia 38: 509–517, 1995
Pierce GN, Dhalla NS: Sarcolemmal Na+-K+-ATPase activity in diabetic rat heart. Am J Physiol 245: C241–C247, 1983
Malhotra A, Penpargkul S, Fein FS, Sonnenblick EH, Scheuer J: The effect of streptozotocin-induced diabetes in rats on cardiac contractile proteins. Circ Res 49: 1243–1250, 1981
Ganguly PK, Pierce GN, Dhalla KS, Dhalla NS: Defective sarcoplasmic reticular calcium transport in diabetic cardiomyopathy. Am J Physiol 244: E528–E535, 1983
Penpargkul S, Schaible T, Yipintsoi T, Scheuer J: The effect of diabetes on performance and metabolism of rat hearts. Circ Res 47: 911–921, 1980
Pierce GN, Kutryk MJ, Dhalla NS: Alterations in Ca2+ binding by and composition of the cardiac sarcolemmal membrane in chronic diabetes. Proc Natl Acad Sci USA 80: 5412–5416, 1983
Afzal N, Dhalla NS: Differential changes in left and right ventricular SR calcium transport in congestive heart failure. Am J Physiol 262: H868–H874, 1992
Dixon IM, Lee SL, Dhalla NS: Nitrendipine binding in congestive heart failure due to myocardial infarction. Circ Res 66: 782–788, 1990
Pfeffer JM, Pfeffer MA, Braunwald E: Hemodynamic benefits and prolonged survival with long-term captopril therapy in rats with myocardial infarction and heart failure. Circulation 75: I149–I155, 1987
Dillmann WH: Influence of thyroid hormone administration on myosin ATPase activity and myosin isoenzyme distribution in the heart of diabetic rats. Metabolism 31: 199–204, 1982
Effron MB, Bhatnagar GM, Spurgeon HA, Ruano-Arroyo G, Lakatta EG: Changes in myosin isoenzymes, ATPase activity, and contraction duration in rat cardiac muscle with aging can be modulated by thyroxine. Circ Res 60: 238–245, 1987
Fiske CH, Subbarow Y: The colorimetric determination of phosphorus. J Biol Chem 66: 375–400, 1925
Caforio AL, Grazzini M, Mann JM, Keeling PJ, Bottazzo GF, McKenna WJ, Schiaffino S: Identification of alpha-and beta-cardiac myosin heavy chain isoforms as major autoantigens in dilated cardiomyopathy. Circulation 85: 1734–1742, 1992
Cummins P, Lambert SJ: Myosin transitions in the bovine and human heart. A developmental and anatomical study of heavy and light chain subunits in the atrium and ventricle. Circ Res 58: 846–858, 1986
Wang J, Liu X, Ren B, Rupp H, Takeda N, Dhalla NS: Modification of myosin gene expression by imidapril in failing heart due to myocardial infarction. J Mol Cell Cardiol 34: 847–857, 2002
Chomczynski P, Sacchi N: Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162: 156–159, 1987
Tso JY, Sun XH, Kao TH, Reece KS, Wu R: Isolation and characterization of rat and human glyceraldehyde-3-phosphate dehydrogenase cDNAs: Genomic complexity and molecular evolution of the gene. Nucleic Acids Res 13: 2485–2502, 1985
Liu X, Takeda N, Dhalla NS: Myosin light-chain phosphorylation in diabetic cardiomyopathy in rats. Metabolism 46: 71–75, 1997
Rupp H, Elimban V, Dhalla NS: Diabetes-like action of intermittent fasting on sarcoplasmic reticulum Ca2+-pump ATPase and myosin isoenzymes can be prevented by sucrose. Biochem Biophys Res Commun 164: 319–325, 1989
Liu X, Sentex E, Golfman L, Takeda S, Osada M, Dhalla NS: Modification of cardiac subcellular remodelling due to pressure overload by captopril and losartan. Clin Exp Hypertens 21: 145–156, 1999
Anthonio RL, van Veldhuisen DJ, van Gilst WH: Left ventricular dilatation after myocardial infarction: ACE inhibitors, beta-blockers, or both? J Cardiovasc Pharmacol 32(suppl 1): S1–S8, 1998
Fiordaliso F, Li B, Latini R, Sonnenblick EH, Anversa P, Leri A, Kajstura J: Myocyte death in streptozotocin-induced diabetes in rats in angiotensin II-dependent. Lab Invest 80: 513–527, 2000
Dandona P, Thusu K, Cook S, Snyder B, Makowski J, Armstrong D, Nicotera T: Oxidative damage to DNA in diabetes mellitus. Lancet 347: 444–445, 1996
Tanaka Y, Gleason CE, Tran PO, Harmon JS, Robertson RP: Prevention of glucose toxicity in HIT-T15 cells and Zucker diabetic fatty rats by antioxidants. Proc Natl Acad Sci USA 96: 10857–10862, 1999
Kajstura J, Fiordaliso F, Andreoli AM, Li B, Chimenti S, Medow MS, Limana F, Nadal-Ginard B, Leri A, Anversa P: IGF-1 overexpression inhibits the development of diabetic cardiomyopathy and angiotensin II-mediated oxidative stress. Diabetes 50: 1414–1424, 2001
Heart Outcomes Prevention Evaluation (HOPE) Study Investigators: Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: Results of the HOPE study and MICRO-Hope substudy. Lancet 355: 253–259, 2000
Berry C, Hamilton CA, Brosnan MJ, Magill FG, Berg GA, McMurray JJ, Dominiczak AF: Investigation into the sources of superoxide in human blood vessels: Angiotensin II increases superoxide production in human internal mammary arteries. Circulation 101: 2206–2212, 2000
Frustaci A, Kajstura J, Chimenti C, Jakoniuk I, Leri A, Maseri A, Nadal-Ginard B, Anversa P: Myocardial cell death in human diabetes. Circ Res 87: 1123–1132, 2000
Fukai T, Siegfried MR, Ushio-Fukai M, Griendling KK, Harrison DG: Modulation of extracellular superoxide dismutase expression by angiotensin II and hypertension. Circ Res 85: 23–28, 1999
Leri A, Claudio PP, Li Q, Wang X, Reiss K, Wang S, Malhotra A, Kajstura J, Anversa P: Stretch-mediated release of angiotensin II induces myocyte apoptosis by activating p53 that enhances the local renin-angiotensin system and decreases the Bcl-2-to-Bax protein ratio in the cell. J Clin Invest 101: 1326–1342, 1998
Liu Y, Leri A, Li B, Wang X, Cheng W, Kajstura J, Anversa P: Angiotensin II stimulation in vitro induces hypertrophy of normal and postinfarcted ventricular myocytes. Circ Res 82: 1145–1159, 1998
Rajagopalan S, Kurz S, Munzel T, Tarpey M, Freeman BA, Griendling KK, Harrison DG: Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation. Contribution to alterations of vasomotor tone. J Clin Invest 97: 1916–1923, 1996
von Harsdorf R, Li PF, Dietz R: Signaling pathways in reactive oxygen species-induced cardiomyocyte apoptosis. Circulation 99: 2934–2941, 1999
Zuanetti G, Latini R, Maggioni AP, Franzosi M, Santoro L, Tognoni G: Effect of the ACE inhibitor lisinopril on mortality in diabetic patients with acute myocardial infarction: Data from the GISSI-3 study. Circulation 96: 4239–4245, 1997
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Machackova, J., Liu, X., Lukas, A. et al. Renin–angiotensin blockade attenuates cardiac myofibrillar remodelling in chronic diabetes. Mol Cell Biochem 261, 271–278 (2004). https://doi.org/10.1023/B:MCBI.0000028765.89855.73
Issue Date:
DOI: https://doi.org/10.1023/B:MCBI.0000028765.89855.73