Abstract
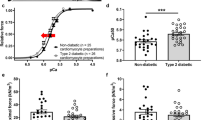
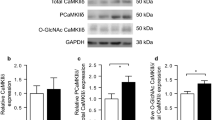
The sarcoplasmic reticulum (SR) plays a critical role in mediating cardiac contractility and its function is abnormal in the diabetic heart. However, the mechanisms underlying SR dysfunction in the diabetic heart are not clear. Because protein phosphorylation regulates SR function, this study examined the phosphorylation state of phospholamban, a key SR protein that regulates SR calcium (Ca2+) uptake in the heart. Diabetes was induced in male Sprague-Dawley rats by an injection of streptozotocin (STZ; 65 mg kg−1 i.v.), and the animals were humanely killed after 6 weeks and cardiac SR function was examined. Depressed cardiac performance was associated with reduced SR Ca2+-uptake activity in diabetic animals. The reduction in SR Ca2+-uptake was consistent with a significant decrease in the level of SR Ca2+-pump ATPase (SERCA2a) protein. The level of phospholamban (PLB) protein was also decreased, however, the ratio of PLB to SERCA2a was increased in the diabetic heart. Depressed SR Ca2+-uptake was also due to a reduction in the phosphorylation of PLB by the Ca2+-calmodulin-dependent protein kinase (CaMK) and cAMP-dependent protein kinase (PKA). Although the activities of the SR-associated Ca2+-calmodulin-dependent protein kinase (CaMK), cAMP-dependent protein kinase (PKA) were increased in the diabetic heart, depressed phosphorylation of PLB could partly be attributed to an increase in the SR-associated protein phosphatase activities. These results suggest that there is increased inhibition of SERCA2a by PLB and this appears to be a major defect underlying SR dysfunction in the diabetic heart. (Mol Cell Biochem 261: 245–249, 2004)
Similar content being viewed by others
References
Barry WH, Bridge JHB: Intracellular calcium homeostasis in cardiac myocytes. Circulation 87: 1806–1815, 1993
Eisner DA, Choi HS, Diaz ME, O'Neill SC, Trafford AW: Integrative analysis of calcium cycling in cardiac muscle. Circ Res 87: 1087–1094, 2000
Bers DM: Ca transport during contraction and relaxation in mammalian ventricular muscle. In: G. Hasenfuss, H. Just (eds). Alterations of Excitation-Contraction Coupling in the Failing Human Heart. Springer-Verlag, New York, 1998: pp 1–16
Bassani JW, Bassani RA, Bers DM: Relaxation in rabbit and rat cardiac cells: Species-dependent differences in cellular mechanisms. J Physiol (Lond) 476: 279–293, 1994
MacLennan DH, Kranias EG: Phospholamban: A crucial regulator of cardiac contractility. Nat Rev Mol Cell Biol 4: 566–577, 2003
Regan TJ, Ahmed S, Haidar B, Moschos C, Weisse A: Diabetic cardiomyopathy: Experimental and clinical observations. N Engl J Med 91: 776–778, 1994
Dhalla NS, Liu X, Panagia V, Takeda N: Subcellular remodeling and heart dysfunction in chronic diabetes. Cardiovasc Res 40: 239–247, 1998
Lagadic-Gossmann D, Buckler KJ, Le Prigent K, Feuvray D: Altered Ca2+-handling in ventricular myocytes isolated from diabetic rats. Am J Physiol 270: H1529–H1537, 1996
Ishikawa T, Kajiwara H, Kurihara S: Alterations in contractile properties and Ca2+-handling in strepzotocin-induced diabetic rat myocardium. Am J Physiol 277: H2185–H2194, 1999
Ganguly PK, Pierce GN, Dhalla KS, Dhalla NS: Defective sarcoplasmic reticular calcium transport in diabetic cardiomyopathy. Am J Physiol 244: E528–E535, 1983
Yu Z, Tibbits GF, McNeill JH: Cellular functions of diabetic cardiomyocytes: Contractility, rapid-cooling contracture, and ryanodine binding. Am J Physiol 266: H2082–H2089, 1994
Netticadan T, Temsah RM, Kent A, Elimban V, Dhalla NS: Depressed levels of Ca2+-cycling proteins may underlie sarcoplasmic reticulum dysfunction in the diabetic heart. Diabetes. 50: 2133–2138, 2001
Netticadan T, Temsah R, Osada M, Dhalla NS: Status of Ca2+/calmodulin protein kinase phosphorylation of cardiac SR proteins in ischemia-reperfusion. Am J Physiol 277: C384–C391, 1999
Osada M, Netticadan T, Tamura K, Dhalla NS: Modification of ischemia-reperfusion-induced changes in cardiac sarcoplasmic reticulum by preconditioning. Am J Physiol 274: H2025–H2034, 1998
Temsah RM, Netticadan T, Chapman D, Takeda S, Mochizuki S, Dhalla NS: Alterations in sarcoplasmic reticulum function and gene expression in the ischemic-reperfused rat heart. Am J Physiol 277: H584–H594, 1999
Netticadan T, Temsah R, Kawabata K, Dhalla NS: Sarcoplasmic reticulum Ca2+/calmodulin protein kinase is altered in heart failure. Circ Res 86: 596–605, 2000
Zhong Y, Ahmed S, Grupp IL, Matlib MA: Altered SR protein expression associated with contractile dysfunction in diabetic rat hearts. Am J Physiol Heart Circ Physiol 281: H1137–H1147, 2001
Pierce GN, Dhalla NS: Cardiac myofibrillar ATPase activity in diabetic rats. J Mol Cell Cardiol 13: 1063–1069, 1981
Makino N, Dhalla KS, Elimban V, Dhalla NS: Sarcolemmal Ca2+ transport in streptozotocin-induced diabetic cardiomyopathy in rats. Am J Physiol 253: E202–E207, 1987
Xu A, Hawkins C, Narayanan N: Phosphorylation and activation of the Ca2+-pumping ATPase of the cardiac sarcoplasmic reticulum by Ca2+/calmodulin-dependent protein kinase. J Biol Chem 263: 1353–1357, 1993
Davis BA, Schwartz A, Samaha FJ, Kranias EG: Regulation of cardiac sarcoplasmic reticulum calcium transport by calcium-calmodulin-dependent phosphorylation. J Biol Chem 258: 13587–13591, 1983
Kargacin ME, Ali Z, Kargacin G: Anti-phospholamban and protein kinase A alter the Ca2+ sensitivity and maximum velocity of Ca2+ uptake by the cardiac sarcoplasmic reticulum. Biochem J 331: 245–249, 1998
LePeuch CJ, Haiech J, Demaille JG: Concerted regulation of cardiac sarcoplasmic reticulum by cyclic adenosine monophosphate dependent and calcium-calmodulin-dependent phosphorylation. Biochemistry 18: 5150–5157, 1979
Kranias EG, Schwartz A, Jungmann RA: Characterization of cyclic 3′,5′-AMP-dependent protein kinase in sarcoplasmic reticulum and cytosol of canine myocardium. Biochim Biophys Acta 709: 28–37, 1982
Baltas LG, Karczewski P, Krause E-G: The cardiac sarcoplasmic reticulum phospholamban kinase is a distinct δ-CaM kinase isozyme. FEBS Lett 373: 71–75, 1995
Yang JM, Cho CH, Kong KA, Jang IS, Kim HW, Juhnn YS: Increased expression of Gáq protein in the heart of strepzotocin-induced diabetic rats. Exp Mol Med 31: 179–184, 1999
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Vasanji, Z., Dhalla, N.S. & Netticadan, T. Increased inhibition of SERCA2 by phospholamban in the type I diabetic heart. Mol Cell Biochem 261, 245–249 (2004). https://doi.org/10.1023/B:MCBI.0000028762.97754.26
Issue Date:
DOI: https://doi.org/10.1023/B:MCBI.0000028762.97754.26