Abstract
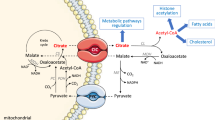
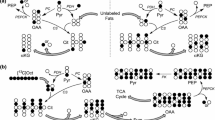
A method to study the export of citric acid cycle intermediates from rat liver mitochondria supplied with various individual substrates or combinations of substrates was designed to focus on the role of mitochondria in anaplerosis and cataplerosis. Under most conditions malate, citrate, and aspartate were exported in far higher amounts than isocitrate and α-ketoglutarate. In the presence of pyruvate alone or pyruvate in combination with most other substrates, citrate export equaled or was only slightly less than malate export. This contrasts with pancreatic islet mitochondria where citrate export is unaffected by many substrates. Malate and succinate potentiated pyruvate-induced citrate export and succinate caused massive malate export from liver mitochondria. Heart mitochondria, which possess very little or no pyruvate carboxylase, unlike liver and pancreatic islet mitochondria, did not produce malate from pyruvate. Heart mitochondria produced malate, but not citrate, from succinate. The results indicate that liver mitochondria export a larger number of metabolites from a wider range of substrates than do islet or heart mitochondria. This may reflect the multiple roles of the liver in body metabolism versus the specialized roles of the islet cell and heart.
Similar content being viewed by others
References
MacDonald MJ: Feasibility of a mitochondrial pyruvate malate shuttle in pancreatic islets. J Biol Chem 270: 20051–20058, 1995
Owen OE, Kalhan SC, Hanson RW: The key role of anaplerosis and cataplerosis for citric acid cycle function. J Biol Chem 277: 30409–30412, 2002
Walter P, Paetkau V, Lardy HA: Paths of carbon in gluconeogenesis and lipogenesis. 3. The role and regulation of mitochondrial processes involved in supplying precursors of phosphoenolpyruvate. J Biol Chem 241: 2523–2532, 1966
Bobyleva V, Kneer N, Bellei M, Battelli D, Lardy HA: Concerning the mechanism of increased thermogenesis in rats treated with dehydroepiandrosterone. J Bioenergetics Biomembr 25: 313–332, 1993
Lardy HA, Paetkau V, Walter P: Paths of carbon in gluconeogenesis and lipogenesis: the role of mitochondria in supplying precursors of phosphoenolpyruvate. Proc Natl Acad Sci USA 53: 1410–1415, 1965
Wiese TJ, Wuensch SA, Ray PD: Synthesis of citrate from phosphoenolpyruvate and acetylcarnitine by mitochondria from rabbit, pigeon and rat liver: Implications for lipogenesis. Comp Biochem Physiol 114B: 417–422, 1996
MacDonald MJ, Chang C-M: Do pancreatic islets contain significant amounts of phosphoenol-pyruvate carboxykinase or ferroactivator activity? Diabetes 34: 246–250, 1985
MacDonald, MJ, McKenzie DI, Walker TM, Kaysen JH: Lack of glyconeogenesis in pancreatic islets: Expression of gluconeogenic enzyme genes in islets. Hormone Metab Res 24: 158–160, 1992
MacDonald MJ: Metabolism of the insulin secretagogue methyl succinate by pancreatic islets. Arch Biochem Biophys 300: 201–205, 1993
MacDonald MJ: Estimates of glycolysis, pyruvate (De)carboxylation, pentose phosphate pathway and methyl succinate metabolism in incapacitated pancreatic islets. Arch Biochem Biophys 305: 205–214, 1993
Khan A, Ling ZC, Landau BR: Quantifying the carboxylation of pyruvate in pancreatic islets. J Biol Chem 271: 2539–2542, 1996
Lu D, Mulder H, Zhao P, Burgess SC, Jensen MV, Kamzolova S, Newgard CB, Sherry AD: 13C NMR isotopomer analysis reveals a connection between pyruvate cycling and glucose-stimulated insulin secretion (GSIS). Proc Natl Acad Sci 99: 2708–2713, 2002
MacDonald MJ: The export of metabolites from mitochondria and anaplerosis in insulin secretion. Biochim Biophys Acta 1619: 77–88, 2003
Davis EJ, Spydevoid O, Bremer J: Pyruvate carboxylase and propionyl-CoA carboxylase as anaplerotic enzymes in skeletal muscle mitochondria. Eur J Biochem 110: 255–262, 1980
Henslee JG, Srere PA: Resolution of rat mitochondrial matrix proteins by two-dimensional polyacrylamide gel electrophoresis. J Biol Chem 254: 5488–5497, 1979
MacDonald MJ: Evidence for the malate-asparate shuttle in pancreatic islets. Arch Biochem Biophys 213: 643–649, 1982
Johnson D, Lardy HA: Isolation of liver or kidney mitochondria. Meth Enzymol 10: 94–96, 1967
Passonneau JV, Lowry OH: In: Enzymatic Analysis, A Practical Guide. Humana Press, Totowa, NJ, 1993
Bergmeyer HU, Bergmeyer J, Grassl M: In: Methods of Enzymatic Analysis, 3rd edn, vol. 7; Metabolites 2: VCH, Verlagsgesellshaft, Weinheim, FRG, 1984
MacDonald MJ, Neufelt N, Park B, Berger M, Ruderman NB: Alanine metabolism and glucoenogenesis in the rat. Am J Physiol 231: 619–626, 1976
MacDonald MJ, Huang MT, Lardy HA: Hyperglycemic activity and metabolic effects of 3-aminopicolinic acid. Biochem J 176: 495–504, 1978
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ: Protein measurement with the Folin phenol reagent. J Biol Chem 193: 265–275, 1951
MacDonald MJ: Differences between mouse and rat pancreatic islets: Succinate responsiveness, malic enzyme and anaplerosis. Am J Physiol Endo Metab 283: E302–E310, 2002
Atkinson DE: In: J.M. Lowenstein (ed). Citric Acid Cycle: Control and Compartmentation. Marcel Dekker, New York, 1969, pp 137–162
MacDonald MJ, Al-Masri H, Jumelle-Laclau M, Cruz M: Oscillations in activities of enzymes in pancreatic islet subcellular fractions induced by physiological concentrations of effectors. Diabetes 46: 1996–2001, 1997
Fahien LA, MacDonald MJ, Kmiotek EH, Mertz RJ, Fahien CM: Regulation of insulin release by factors that also modify glutamate dehydrogenase. J Biol Chem 263: 13610–13614, 1988
Gylfe E: Comparison of the effects of leucines, non-metabolizable leucine analogues and other insulin secretagogues on the activity of glutamate dehydrogenase. Acta Diabetol Lat 13: 20–24, 1976
Malaisse-Lagae F, Sener A, Garcia-Morales P, Valverde I, Malaisse WJ: The stimulus-secretion coupling of amino acid-induced insulin release. Influence of a nonmetabolized analog of leucine on the metabolism of glutamine in pancreatic islets. J Biol Chem 257: 3754–3758, 1982
Lenzen S, Schmidt W, Panten U: Transamination of neutral amino acids and 2-keto acids in pancreatic B-cell mitochondria. J Biol Chem 260: 12629–12634, 1985
MacDonald MJ, Fahien LA: Glutamate is not a messenger in insulin secretion. J Biol Chem 275: 34025–34027, 2000
Fahien LA, Teller JK: Glutamate-malate metabolism in liver mitochondria. A model constructed on the basis of mitochondrial levels of enzymes, specificity, dissociation constants, and stoichiometry of hetero-enzyme complexes. J Biol Chem 267: 10411–10422, 1992
Williamson DH, Lund P, Krebs HA: The redox state of free nicotinamide-adenine dinucleotide in the cytoplasm and mitochondria of rat liver. Biochem J 103: 514–527, 1967
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
MacDonald, M.J. Production and export of metabolites from liver and heart mitochondria and anaplerosis. Mol Cell Biochem 258, 201–210 (2004). https://doi.org/10.1023/B:MCBI.0000012856.31929.91
Issue Date:
DOI: https://doi.org/10.1023/B:MCBI.0000012856.31929.91