Abstract
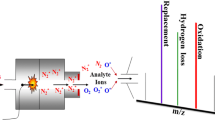
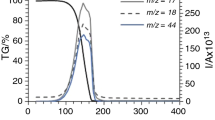
Identification and monitoring of gaseous species released during thermal decomposition of the title compound 1, Zn(tu)2Cl2, (tu=thiourea, (NH2)2C=S) have been carried out in flowing air atmosphere up to 800°C by both online coupled TG-EGA-FTIR and simultaneous TG/DTA-EGA-MS. The first gaseous products of 1, between 200 and 240°C, are carbon disulfide (CS2) and ammonia (NH3). At 240°C, an exothermic oxidation of CS2 vapors occurs resulting in a sudden release of sulphur dioxide (SO2) and carbonyl sulphide (COS). An intense evolution of hydrogen cyanide (HCN) and beginning of the evolution of cyanamide (H2NCN) and isothiocyanic acid (HNCS) are also observed just above 240°C. Probably because of condensation and/or polymerization of cyanamide vapors on the windows and mirrors of the FTIR gas cell optics, some strange baseline shape changes are also occurring above 330°C. Above 500°C the oxidation process of organic residues appears to accelerate which is indicated by the increasing concentration of CO2, while above 600°C zinc sulfide starts to oxidize resulting in the evolution of SO2. All species identified by FTIR gas cell were also confirmed by mass spectrometry, except for HNCS.
Similar content being viewed by others
References
O. Kijatkina, M. Krunks, A. Mere, B. Mahrov and L. Dloczik, Thin Solid Films, 431 (2003)105.
M. Krunks, O. Kijatkina, H. Rebane, I. Oja, V. Mikli and A. Mere, Thin Solid Films, 403 (2002) 71.
M. Krunks, O. Bijakina, V. Mikli, H. Rebane, T. Varema, M. Altosaar and E. Mellikov, Solar Energy Mater. Solar Cells, 69 (2001) 93.
T. Fujiwara, M. Okuya and S. Kaneko, J. Ceram. Soc. Japan, 110 (2002) 81.
M. Krunks, J. Madarász, L. Hiltunen, R. Mannonen, E. Mellikov and L. Niinistö, Acta Chem Scand., 51 (1997)294.
M. Krunks, T. Leskelä, R. Mannonen and L. Niinistö, J. Therm. Anal. Cal., 53 (1998)355.
J. Madarász, P. Bombicz, M. Okuya, S. Kaneko and G. Pokol, Solid State Ionics, in press.
J. Madarász, P. Bombicz, M. Okuya, S. Kaneko and G. Pokol, J. Anal. Appl. Pyrol., 2004, 72 (2004)209.
J. Madarász, P. Bombicz, M. Okuya and S. Kaneko, Solid State Ionics, 141-142 (2001)439.
M. Krunks, J. Madarász, T. Leskelä A. Mere, G. Pokol and L. Niinistö, J. Therm. Anal. Cal., 71 (2003)421.
NIST Chemistry Webbook Standard Reference Database No. 69, March,2003 Release, EPA Vapor Phase Library.
CRC Handbook of Chemistry and Physics, 65th Edition, CRC Press, 1985, p. B-159.
T. Dedova, A. Mere, M. Krunks, O. Kijatkina, I. Oja and O. Volobujeva, Proceedings of SPIE, Advanced Organic and Inorganic Optical Materials, submitted.
L. Niinistö, J. Therm. Anal. Cal., 56 (1999)7.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Madarász, J., Krunks, M., Niinistö, L. et al. Evolved gas analysis of dichlorobis(thiourea)zinc(II) by coupled TG-FTIR and TG/DTA-MS techniques. Journal of Thermal Analysis and Calorimetry 78, 679–686 (2004). https://doi.org/10.1023/B:JTAN.0000046127.69336.90
Issue Date:
DOI: https://doi.org/10.1023/B:JTAN.0000046127.69336.90