Abstract
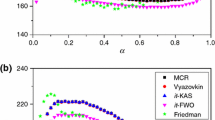
A new method of the multiple rate iso-temperature was used to define the most probable mechanism g(α) of a reaction; the iterative iso-conversional procedure has been employed to estimate apparent activation energy E a, the pre-exponential factor A was obtained on the basis of E a and g(α). In this new method, the thermal analysis kinetics triplet of dehydration of calcium oxalate monohydrate is determined, which apparent activation energy E a is 82.83 kJ mol-1, pre-exponential factor A is 1.142·105-1.235·105 s-1, the most probable mechanism belongs to phase boundary reaction Rn with integral form g(α)=1-(1-α)n and differential form f(α)=n(1-α)1-(1/n), where accommodation factor n=2.40-1.40.
Similar content being viewed by others
References
B. Malecka, E. Drozdz-Ciesla and Malecki, J. Therm. Anal. Cal., 68 (2002) 819.
K. E. Ozbas, M. V. Kök and C. Hicyilmaz, J. Therm. Anal. Cal., 71 (2003) 849.
J. P. Elder, Thermochim. Acta, 318 (1998) 229.
J. Zsakó, I. Szilágyi and A. Simay, J. Therm. Anal. Cal., 69 (2002) 125.
A. W. Coats and J. P. Redfern, Nature (London), 201 (1964) 68.
B. N. Achar, G. W. Brindley and J. H. Sharp, Proceedings of International Clay Conference, 1 (1966) 67.
C. Popescu, Thermochim. Acta, 285 (1996) 309.
Z. Gao, I. Amasaki and M. Nakada, Thermochim. Acta, 385 (2002) 95.
X. Gao and D. Dollimore, Thermochim. Acta, 215 (1993) 47.
R. Z. Hu and Q. Zh. Shi, "Thermal Analysis Kinetics', Science Press, Beijing 2001, p. 20.
Z. Gao, M. Nakada and I. Amasaki, Thermochim. Acta, 369 (2001) 137.
Z. H. Liu, "Introduction to Thermal Analysis', Chemistry-Industry Press, Beijing 1991, p. 73.
H. E. Kissinger, Anal. Chem., 29 (1957) 1702.
P. Budrugeac, D. Homentcovschi and E. Segal, J. Therm. Anal. Cal., 66 (2001) 562.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Liqing, L., Donghua, C. Application of iso-temperature method of multiple rate to kinetic analysis. Journal of Thermal Analysis and Calorimetry 78, 283–293 (2004). https://doi.org/10.1023/B:JTAN.0000042175.27569.ee
Issue Date:
DOI: https://doi.org/10.1023/B:JTAN.0000042175.27569.ee