Abstract
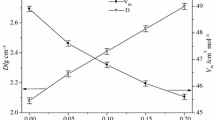
The relation between change of the specific heat (ΔC p) accompanying the glass transformation and the chemical composition of glasses (Na2O, CaO, MgO)-Al2O3-SiO2 system has been studied. The exchange of modifiers in the glass structure causes the ΔC p increase in the sequence Na>Ca>Mg. Change the glass network composition by introducing Al into it makes smaller increase of the ΔC p values. It has been shown that degree of ΔC p value changes is dependent on the iconicity/covalence of chemical bonds of cations with oxygen of glass structure network.
Similar content being viewed by others
References
L. Stoch, J. Therm. Anal. Cal., 54 (1998) 9.
L. Stoch, J. Therm. Anal. Cal., 77 (2004) 7.
I. Wacławska, M. Środa and L. Stoch, J. Therm. Anal. Cal., 65 (2001) 661.
L. Pauling, Nature of the chemical bonds, Cornell Univ. Press. Ithaca 1950.
E. Görlich, Z. Phys. Chem., 271 (1990) 169.
L. Stoch and M. Środa, J. Mol. Struct., 511–512 (1999) 77.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Stoch, L., Wacławska, I. & Środa, M. Thermal study of the influence of chemical bond ionicity on the glass transformation in (Na2O, CaO, MgO)-Al2O3-SiO2 glasses. Journal of Thermal Analysis and Calorimetry 77, 57–63 (2004). https://doi.org/10.1023/B:JTAN.0000033188.21587.6d
Issue Date:
DOI: https://doi.org/10.1023/B:JTAN.0000033188.21587.6d