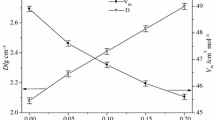
Abstract
Thermochemistry and structural mechanism of crystallization of MgO-Al2O3-SiO2 glasses with TiO2 as crystallization activator were studied. Thermal and HREM investigation proved that near the T g temperature crystallization is going by rearrangement of glass structure elements and part of its components redistribution like at disorder — order phase transition in solid bodies. Nanocrystals of Mg-titanate and high quartz structure solid solution are formed then. Next enstatite and cordierite are crystallizing. Thermochemical and chemical bonds strength analysis indicate that during multistage crystallization of glasses, kind and order of crystal phase formation, is determined by the glass structure decomposition progress and its particular components release accompanying increase of temperature. It has been proved that molar heat capacity change (ΔC p) accompanying the glass transition is the significant measure of degree of changes in the structure of glass preceding crystallization.
Similar content being viewed by others
References
L. Stoch, in Proceedings of the 19th International Congress on Glass Edinbourgh, Scotland, I. Society of Glass of Glass Technology, Sheffield, 2001, v. 1. 62.
L. R. Pinckney and G. H. Beall, J. Non-Cryst. Solids, (1997) 219.
I. Wacławska and M. Szumera, J. Therm. Anal. Cal., 72 (2003) 1065.
M. Ciecinska, J. Therm. Anal. Cal., 72 (2003) 199.
I. Gutzow and J. Schmelzer, The vitreous state, Springer-Verlag, Berlin, Heidelberg, 1995.
L. Stoch, J. Therm. Anal. Cal., 54 (1998) 9.
I. Wacławska, L. Stoch and M. Środa, J. Therm. Anal. Cal. (this issue).
L. Stoch, J. Thermal Anal., 48 (1997) 121.
I. Prigogine and R. Defay, Chemical thermodynamics. Longmans Green, London 1954.
E. Görlich, Z. Phys. Chem., 271 (1990) 169.
I. Barin, The thermodynamic data of pure substances. VCH Verlagsgesellschaft, Weinheim, 1993.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Stoch, L. Thermal analysis and thermochemistry of vitreous into crystalline state transition. Journal of Thermal Analysis and Calorimetry 77, 7–16 (2004). https://doi.org/10.1023/B:JTAN.0000033182.90571.ce
Issue Date:
DOI: https://doi.org/10.1023/B:JTAN.0000033182.90571.ce