Abstract
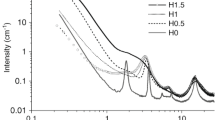
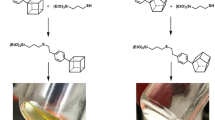
A new and general route to synthesize shape-controlled bridged silsesquioxanes has been developed by the hydrolysis-condensation of molecularly designed precursors bearing urea groups. The auto-association of the bridging organic units, owing to the hydrogen bonds developing between the urea groups, has been exploited to create new multifunctional organo-bridged silicas with peculiar shapes from the nano- to the micro-scale. Helical fibers with controlled handedness, hollow tubes and spheres, and lamellar plates have been produced according to the main organic substructure and also depending on the reaction conditions (catalyst, solvent and temperature). Spectroscopic techniques (solid state 13C and 29Si NMR, FTIR and X-ray diffraction) and electronic microscopic measurements (SEM and TEM) allowed the characterisation of these hybrid materials.
Similar content being viewed by others
References
K.J. Shea, D.A. Loy, and O.W. Webster, Chem. Mater. 1, 512 (1989); K.J. Shea, D.A. Loy, and O.W. Webster, J. Amer. Chem. Soc. 114, 6700 (1992); K.J. Shea and D.A. Loy, Chem. Rev. 95, 1431 (1995).
R.J.P. Corriu, J.J.E. Moreau, P. Th´epot, and M. Wong Chi Man, Chem. Mater. 4, 1217 (1992); R.J.P. Corriu and D. Leclerc, Angew. Chem., Int. Ed. Engl. 35, 1420 (1996); J.J.E. Moreau and M. Wong Chi Man, Coord. Rev. 178-180, 1073 (1998); R.J.P. Corriu, Angew. Chem., Int. Ed. Engl. 39, 1376 (2000).
C.J. Brinker and G.W. Scherer, Sol-Gel Science: The Physics and Chemistry of Sol-Gel Processing (Academic Press, 1990).
C. Sanchez and F. Ribot, New J. Chem. 18, 1007 (1994); P. Judeinstein and C. Sanchez, J. Mater. Chem. 6, 511 (1996).
U. Schubert, New J. Chem. 18, 1049 (1994); U. Schubert, N. H¨using, and A. Lorenz, Chem. Mater. 7, 2012 (1995).
D. Avnir, Acc. Chem. Res. 28, 328 (1995).
C. Sanchez, G.J. de A.A. Soler-Illia, F. Ribot, C.R. Mayer, and V. Cabuil, Chem. Mater. 13, 3061 (2001); G.J. de A.A. Soler-Illia, C. Sanchez, B. Lebeau, and J. Patarin, Chem. Rev. 102, 4093 (2002).
A. Adima, J.J.E. Moreau, and M. Wong Chi Man, J. Mater. Chem. 7, 2331 (1997); A. Adima, J.J.E. Moreau, and M. Wong Chi Man, Chirality 12, 411 (2000); P. Hesemann and J.J.E. Moreau, Tetrahedron: Asymmetry 11, 2183 (2000).
H.W. Oviatt, K.J. Shea, S. Kalluri, Y. Shi, W.H. Steier, and L.R. Dalton, Chem. Mater. 7, 493 (1995).
J.-C. Broudic, O. Conocar, J.J.E. Moreau, D. Meyer, and M. Wong Chi Man, J. Mater. Chem. 9, 2283 (1999); S. Bourg, J.-C. Broudic, O. Conocar, J.J.E. Moreau, D. Meyer, and M. Wong Chi Man, Mater. Res. Soc. Symp. Proc. 628, CC1.6.1 (2000); S. Bourg, J.-C. Broudic, O. Conocar, J.J.E. Moreau, D. Meyer, and M. Wong Chi Man, Chem. Mater. 13, 491 (2001).
C.T. Kresge, M.E. Leonowicz, W.J. Roth, J.C. Vartulli, and J.S. Beck, Nature 359, 710 (1992); J.S. Beck, J.C. Vartulli, W.J. Roth, M.E. Leonowicz, C.T. Kresge, K.D. Schmitt, C.T.-W. Chu, D.H. Olson, E.W. Sheppard, S.B. McCullen, J.B. Higgins, and J.L. Schlenker, J. Am. Chem. Soc. 114, 10834(1992).
S. Inagaki, S. Guan, Y. Fukushima, T. Ohsuna, and O. Terasaki, J. Am. Chem. Soc. 121, 9611 (1999); S. Guan, S. Inagaki, T. Ohsuna, and O. Terasaki, Nature 416, 304 (2002).
Asefa, M.J. MacLachlan, N. Coombs, and G.A. Ozin, Nature 402, 867 (1999); M.J. MacLachlan, T. Asefa, and G.A. Ozin, Chem. Eur. J. 2507 (2000).
B.J. Melde, B.T. Holland, C.F. Blanford, and A Stein, Chem. Mater 11, 3302 (1999).
Y. Lu, H. Fan, N. Doke, D.A. Loy, R.A. Assink, D.A. LaVan, and C.J. Brinker, J. Am. Chem. Soc 122, 5258 (2000).
A. Sayari, S. Hamoudi, Y. Yang, I.L. Moudrakovski, and J.R. Ripmeester, 12, 3857 (2000).
H. Zhu, D.J. Jones, J. Zarzac, J. Rozière, and R. Dutartre, Chem. Commun. 2568 (2001).
V. Goletto, A.-C. Bled, G. Trimmel, M. Wong Chi Man, H.-G. Woo, D. Durand, and F. Babonneau, Mater. Res. Soc. Symp. Proc. 726, Q.6.14.1 (2002).
B. Boury and R.J.P. Corriu, Chemistry Organic Silicon Compounds 3, 565 (2001).
J.J.E. Moreau, L. Vellutini, M. Wong Chi Man, and C. Bied, J. Amer. Chem. Soc. 123, 1509 (2001).
J.J.E. Moreau, L. Vellutini, M. Wong Chi Man, and C. Bied, Chem. Eur. J. 9, 1594 (2003).
J.J.E. Moreau, L. Vellutini, M. Wong Chi Man, C. Bied, J.-L. Bantignies, P. Dieudonn´e, and J.-L. Sauvajol, J. Amer. Chem. Soc. 123, 7957 (2001).
J. van Esch, F. Schoonbeek, M. de Loos, H. Kooijman, A.L. Spek, R.M. Kellogg, and B.L. Feringa, Chem. Eur. J. 5, 937 (1999).
C. Bied, J.J.E. Moreau, L. Vellutini, and M. Wong Chi Man, J. Sol-Gel Sci. Technol. 26, 538 (2003).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Moreau, J.J., Vellutini, L., Bied, C. et al. New Approach for the Organisation and the Shaping of Organo-Bridged Silicas: An Overview. Journal of Sol-Gel Science and Technology 31, 151–156 (2004). https://doi.org/10.1023/B:JSST.0000047977.44966.53
Issue Date:
DOI: https://doi.org/10.1023/B:JSST.0000047977.44966.53