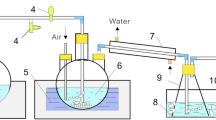
Abstract
The isotope fractionation of potassium in an aqueous (KOH)/amalgam system has been studied. Two types of isotope effects with opposite isotope enrichment directions were observed in the electrolysis of potassium from the aqueous into the amalgam phase under constant electrolytic potentials. It was found that the first isotope effect causing the light isotope enriched in the amalgam is related to the kinetic process of the mass transfer through the aqueous/amalgam interface, while the second one leading to the enrichment of the heavy isotope in the amalgam phase is produced by the isotope-exchange equilibrium. The temperature dependence of the equilibrium isotope effect was also investigated using single-stage and multi-stage techniques. It was observed that the equilibrium isotope effect increases as the temperature increases in the range of 293-371 K. An empirical equation was used to fit the variations of the isotope effects with temperature for potassium together with the other alkaline and alkaline earth metals studied in the same system. The origin of the equilibrium isotope fractionation in the electron-exchange system was discussed. Furthermore, the mass dependence of the separation coefficients of the alkaline and alkaline earth metals observed in aqueous/amalgam and ion-exchange systems were compared. At 293 K the equilibrium isotope separation coefficient for the 39K/41K isotopes in the amalgam system was determined as (5.6±0.6).10-3.
Similar content being viewed by others
References
Z. Chang, M. Hosoe, M. Nomura, Y. Fujii, J. Chem. Soc. Faraday Trans., 91 (1996) 2319.
Z. Chang, M. Nomura, K. Motomiya, Y. Fujii, J. Chem. Soc. Faraday Trans., 92 (1996) 4485.
D. Chen, Z. Chang, M. Nomura, Y. Fujii, J. Chem. Soc. Faraday Trans., 93 (1997) 2395.
A. A. Palko, J. S. Drury, G. M. Begun, J. Chem. Phys., 64 (1976) 1828.
D. Hutchison. J. Chem. Phys., 14 (1946) 401.
H. H. Garretson, J. S. Drury, J. Chem. Phys., 34 (1961) 1957.
K. Okuyama, I. Okada, N. Saito, J. Inorg. Nucl. Chem., 35 (1972) 2883.
M. Fujie, Y. Fujii, M. Nomura, M. Okamoto, J. Nucl. Sci. Technol., 23 (1986) 330.
J. Bigeleisen, J. Am. Chem. Soc., 118 (1996) 3676.
Y. Fujii, M. Nomura, Y Ban, J. Nucl. Sci. Technol., 39 (2002) 413.
E. Michiels, P. D. Bievre, Intern. J. Mass Spectrom. Ion Phys., 49 (1983) 265.
A. J. Bard, L. R. Faulkner, Electrochemistry Methods, John Wiley & Sons, New York, 1980, p. 119.
H. C. Urey, J. Chem. Soc., 15 (1947) 562
J. Bigeleisen, M. G. Mayer, J. Chem. Phys., 15 (1947) 261
L. I. Kleinman, M. Wolfsberg, J. Chem. Phys., 59 (1973) 2043.
T. Ishida, J. Bigelesen, J. Chem. Phys., 64 (1976) 4775.
G. Jancso, L. P. N. Rebelo, W. A. Van Hook, Chem. Rev., 93 (1993) 2645.
Y. Fujii, M. Nomura, M. Okamoto, H. Onitsuka, F. Kawakami, K. Takeda, Z. Naturforsch., 44a (1989) 395.
Y. Fujii, M. Nomura, M. Okamoto, H. Onitsuka, T. Nakanishi, Bulletin Research Laboratory for Nuclear Reactors, Special Issue No. 1, Y. Fujii, T. Ishida and K. Takeuchi (Eds), ISSN 0387-6144, Tokyo, 1992, p. 214.
M. Nomura, N. Higuchi, Y. Fujii, J. Am. Chem. Soc., 118 (1996) 9127.
D. Zuker, J. S. Drury, J. Chem. Phys., 34 (1964) 1957.
T. Oi, K. Kawada, M. Hosoe, H. Kakihana, Separ. Sci. Technol., 26 (1991) 1353.
K. Kawada, T. Oi, M. Hosoe, H. Kakihana, J. Chromatogr. 538 (1991) 355.
M. Hosoe, T. Oi, K. Kawada, H. Kakihana, J. Chromatogr., 438 (1988) 225.
T. Oi, S. Yanase, H. Kakihana, Separ. Sci. Technol., 22 (1987) 2203.
T. Oi, N. Morika, H. Ogino, H. Kakihana, M. Hosoe, Separ. Sci. Technol., 28 (1993) 1971.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Chang, Z., Nomura, M. & Fujii, Y. Isotope effect of potassium in an aqueous/amalgam system. Journal of Radioanalytical and Nuclear Chemistry 258, 511–518 (2003). https://doi.org/10.1023/B:JRNC.0000011744.78574.3a
Issue Date:
DOI: https://doi.org/10.1023/B:JRNC.0000011744.78574.3a