Abstract
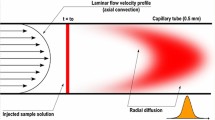
The mean diffusion coefficient of 233Pa has been measured simultaneously with those of 22Na and 152Eu in 0.5 M (Na, H)ClO4 solutions with the pH ranging from 0.3 to 13, by the “open-end capillary method” optimized in order to obtain reproducible and reliable D values at T = 25°C. In the case of Eu(III), the results tend to give higher β13 and β14 hydrolysis constants than the values generally acccepted, but these data are probably affected by the formation of polynuclear or colloidal species as soon as the hydrolysis process is involved. For Pa(V), results are in agreement with the existence of the following two equilibria (I = 0.5 M, T = 25°C):
However, unusual behavior is observed at a pH value around 1.3. A third equilibrium in basic media leads to the formation of a negatively charged species (log K h4 = −9.03 ± 0.1 at I = 0.5 M). Finally, the presence of chloride in solution (0.1 M; pH = 1 and 4) and carbonate-bicarbonate ions (0.1 M; pH = 9.4 and 11.0), which cannot be neglected in most of the natural waters, decreases the measured values for the diffusion coefficient of Pa(V) appreciably compared to noncomplexing media.
Similar content being viewed by others
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Fourest, B., Perrone, J., Tarapcik, P. et al. The Hydrolysis of Protactinium(V) Studied by Capillary Diffusion. Journal of Solution Chemistry 33, 957–973 (2004). https://doi.org/10.1023/B:JOSL.0000048047.62202.55
Issue Date:
DOI: https://doi.org/10.1023/B:JOSL.0000048047.62202.55