Abstract
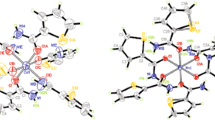
The structure of the diammonium salt of 5-nitraminotetrazole wasestablished bysinglecrystalX-ray diffraction. The crystals are monoclinic, space group P2/c; a=7.272(1), b=8.643(1), c=10.427(1) Å; β=93.20(1)°; V=654.33(39) Å3; Z=4;ρcalc=1.666 g/cm3. The dianion is nonplanar, but contains two practically planar fragments -- the tetrazole cycle and the nitramine group. The ammonium cations form an extended net of hydrogen bonds with the dianions, involving all hydrogen atoms. The negative charges of the dianion are located on the nitro oxygen atoms and the nitrogens of the tetrazole cycle. The difference between the geometrical parameters of the compound and those of other 5-nitraminotetrazole salts is examined.
Similar content being viewed by others
REFERENCES
D. Vasiliev, A.M. Astakhov, A.A. Nefedov, and R.S. Stepanov, Zh.Strukt.Khim., 44, No. 2, 359–363 (2003).
V.A. Ostrovskii and G.I. Koldobskii, Ross.Khim.Zh., 41, No. 2, 84–98 (1977).
A. Gao, A.L. Rheingold,and T.B. Brill, Propellants,Explosives,Pyrotechnics, 16, No. 3, 97–104 (1991).
A.M. Astakhov, R.S. Stepanov, L.A. Kruglyakova,and A.A. Nefedov, Zh.Org.Khim., 37, No. 4, 611–616 (2001).
T.B. Brill, B.C. Tappan,and R.W. Beal, Proc.4th Seminar "New Trends in Research of Energetic Materials," Pardubice, Czech Republic (2001), pp.17–28.
S.F. Palopoli, S.J. Geib, A.L. Rheingold,and T.B. Brill, Inorg.Chem., 27, No. 17, 2963–2971 (1988).
G.M. Sheldrick, SHELX-97,Release 97-2, Univ.Göttingen, Germany (1998).
F. H. Allen and O. Kennard, Chem.Des.Autom.News., 8, No. 1, 31–37 (1993).
C. S. Choi, Acta Crystallogr., B37, 1955–1957 (1981).
S. Nordenson, ibid.,1543–1547.
S. Nordenson and J. Hvoslef, ibid.,373–378.
S. Nordenson, ibid., 1774–1776.
S. Rice, M.Y. Cheng, R.E. Cramer, et al., J.Am.Chem.Soc., 106, No. 19, 239–243 (1984).
Y. Oyumi, A.L. Rheingold,and T.B. Brill, Propellants,Explosives,Pyrotechnics, 12, No. 3, 46–52 (1987).
A.D. Vasiliev, A.M. Astakhov, A.A. Nefedov, et al., Acta Crystallogr., C57, 625/626 (2001).
A. M. Astakhov, I.V. Gelemurzina, A.D. Vasiliev, et al., Energetic Materials --Ignition,Combustion and Detonation, 32nd Int.ICT Conf., Karlsruhe,FRG (2001), P139/1–10.
A.M. Astakhov, A.D. Vasiliev, M.S. Molokeev, et al., Proc.5th Seminar "New Trends in Research of Energetic Materials," Pardubice, Czech Republic (2002),pp.29–41.
N.I. Sadova and L. V. Vilkov, Usp.Khim., 51, No. 1, 153–184 (1982).
Yu.V. Zefirov and P.M. Zorkii, ibid., 58, No. 5, 713–746 (1989).
T. Steiner, IUCr Newsletter, 8, No. 4, 19/20 (2000).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Astakhov, A.M., Vasiliev, A.D., Molokeev, M.S. et al. Crystal and Molecular Structure of Nitramino Derivatives of Tetrazole and 1,2,4-Triazole. II. 5-Nitraminotetrazole Diammonium Salt. Journal of Structural Chemistry 45, 175–180 (2004). https://doi.org/10.1023/B:JORY.0000041520.90323.fe
Issue Date:
DOI: https://doi.org/10.1023/B:JORY.0000041520.90323.fe