Abstract
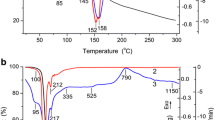
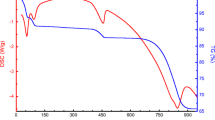
This paper deals with a systematic investigation on the synthesis of aluminium oxide starting from mono hydroxy aluminium oxide (boehmite, (AlOOH)) which in turn is synthesized from aluminium nitrate. Boehmite on heating forms γ, transitional alumina and α-alumina phases in the temperature range 400–600, 800–1050 and 1050–1100°C respectively. Calculation from XRD, using Scherer equation shows that γ-alumina has crystallite size in the range 5–10 nm, while δ and θ-alumina are in the range 10–20 nm and α-alumina is about ∼33 nm. The textural features of aqueous sol-gel boehmite samples calcined at various temperatures were analyzed by specific surface area measurements and adsorption isotherm features. Maximum specific surface area of 266 m2/g is observed for the precursor calcined at 400°C and a minimum of 5 m2/g at 1100°C. Total pore volume is maximum for the precursor calcined at 600°C (0.2653 cm3/g). Average pore size ranges from ∼3 nm (400°C) to ∼11 nm (1100°C). The adsorption isotherms also show a change from Type IV to Type II with increase in temperature showing difference in surface properties. The information from t-plots, pore size distribution and cumulative pore volume data also indicates differences in porosity features of boehmite on calcination. The adsorption isotherm and pore size distribution analysis show maximum microporosity at 400°C, while maximum mesoporosity is observed at 600°C. At higher temperatures, porosity decreases, even though small fraction of pores in the mesopore range is still retained. At 1100°C, there is structural transformation from transitional to α-alumina, with very low specific surface area ∼5 m2/g and pores in the size range of 11 nm. The various data presented in this study will be useful in the synthesis of alumina with tailor made properties.
Similar content being viewed by others
References
D.L. Trimm and A. Stanislus, App. Catal. B21, 215 (1986).
E. Elaloui, A.C. Pierre, and G.M. Pajonk, J. Catal. 166, 340 (1997).
K. Okada, A. Tanaka, S. Hayashi, K. Daimon, and N. Otsuka, J. Mater. Res. 9, 1709 (1994).
T. Fukui and M. Hori, J. Mat. Sci 31, 3245 (1996).
N. Baba, A. Nogami, K. Terasaki, K. Kawasaki, and Y. Ozaki, J. Ceram. Soc. Jpn. 106(1), 65–69 (1998).
G.V.R. Rao, S. Venkadesan, and V. Saraswathi, J. Non-cryst. Solids 111, 103 (1989).
V. Saraswati and G.V.R. Rao, J. Mat. Sci. Lett. 5(11), 1095 (1986).
V. Saraswati and G.V.R. Rao, Bull. Mat. Sci. 9, 193 (1987).
J.A. Wang, X. Bokhimi, A. Morales, O. Novaro, T. Lopez, and R. Gomez, J. Phy. Chem. B 103, 299 (1999).
W.H. Gitzen, Alumina as a Ceramic Material (American Ceramic Society, Columbus, Ohio, 1970).
T.V. Mani, P.K. Pillai, A.D. Damodaran, and K.G.K. Warrier, Mater. Lett. 19, 237 (1994).
S. Lowel and J.E. Shields, Powder Surface Area and Porosity, 2nd edition (Chapman and Hall, London, 1984).
R.Z. Zhou and R. Snyder, Acta Cryst. B 47(5), 617 (1991).
G. Krishnapriya, P. Padmaja, K.G.K. Warrier, A.D. Damodaran, and G. Aruldhas, J. Mater. Sci. Lett. 16, 158 (1997).
G.L. Messing and M. Kumagai, Am. Ceram. Soc. Bull. 73(10), 88 (1994).
B. Be Whitte, K. Vercruysse, K. Aernouts, P. Verwimp, and J.B. Utterhoeven, J. Porous Mater. 2, 307 (1996).
A.C. Pieree, Introduction to Sol-Gel Processing, edited by L.C. Klein (Kluwer Academic Publishers, The Netherlands, 1998).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Padmaja, P., Pillai, P.K., Warrier, K. et al. Adsorption Isotherm and Pore Characteristics of Nano Alumina Derived from Sol-Gel Boehmite. Journal of Porous Materials 11, 147–155 (2004). https://doi.org/10.1023/B:JOPO.0000038010.54859.2f
Issue Date:
DOI: https://doi.org/10.1023/B:JOPO.0000038010.54859.2f