Abstract
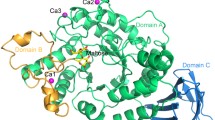
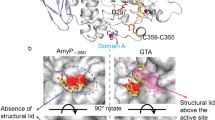
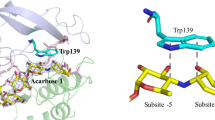
The X-ray structure analysis of a crystal of pig pancreatic α-amylase soaked with a ρ-nitrophenyl-α-D-maltoside (pNPG2) substrate showed a pattern of electron density corresponding to the binding of a ρ-nitrophenol unit at subsite −2 of the active site. Binding of the product to subsite −2 after hydrolysis of the pNPG2 molecules, may explain the low catalytic efficiency of the hydrolysis of pNPG2 by PPA. Except a small movement of the segment from residues 304–305 the typical conformational changes of the “flexible loop” (303–309), that constitutes the surface edge of the substrate binding cleft, were not observed in the present complex structure. This result supports the hypothesis that significant movement of the loop may depend on aglycone site being filled (Payan and Qian, J. Protein Chen. 22: 275, 2003). Structural analyses have shown that pancreatic α-amylases undergo an induced conformational change of the catalytic residue Asp300 upon substrate binding; in the present complex the catalytic residue is observed in its unliganded orientation. The results suggest that the induced reorientation is likely due to the presence of a sugar unit at subsite −1 and not linked to the closure of the flexible surface loop. The crystal structure was refined at 2.4 Å resolution to an R factor of 17.55% (R free factor of 23.32%).
Similar content being viewed by others
REFERENCES
Ajandouz, E. H., Abe, J., Svensson, B., and Marchis-Mouren, G. (1992). Biochim. Biophys. Acta. 1159: 193–202.
Ajandouz, E. H. and Marchis-Mouren, G. J. (1995). Carbohydr. Res. 268: 267–277.
Brayer, G. D., Luo, Y., and Withers, S. G. (1995). Protein Sci. 4: 1730–1742.
Brayer, G. D., Sidhu, G., Maurus, R., Rydberg, E. H., Braun, C., Wang, Y., Nguyen, N. T., Overall, C. M., and Withers, S. G. (2000). Biochemistry 39: 4778–4791.
BrÝnger, A. T. (1992). Nature 355: 472–475.
BrÝnger, A. T. (1996). XPLOR verion 3.843 Manural. New Haven, Connecticut: Yale University Press.
BrÝnger, A. T., Kuriyan, J., and Karplus, M. (1987). Science 35: 458–460.
Coutinho, P. M. and Henrissat, B. (1999) in Recent Advances in Carbohydrate Bioengineering., ed. Gilbert, H. J., Davies, G. J., Henrissat, B. and Svensson, B. (The Royal Society, Cambridge).
Davies, G. J., Wilson K. S., and Henrissat, B. (1997). Biochem. J. 321: 557–559.
Ishikawa, K., Matsui, I., and Honda, K. (1990). Biochemistry 29: 7119–7123.
Koshland, D. E. (1953). Biol. Rev. 28: 416–436.
Larson, S. B., Greenwood, A., Cascio, D., Day, J., and McPherson, A. (1994). J. Mol. Biol. 235: 1560–1584.
MacGregor, A. W., Morgan, J. E., and MacGregor, E. A. (1992). Carbohydr. Res. 227: 301–313.
McCarter, J. D. and Withers, S. G. (1994). Curr. Opin. Struct. Biol. 4: 885–892.
Nahoum, V., Roux, G., Anton, V., Rougeé, P., Puigserver, A., Bischoff, H., Henrissat, B., and Payan, F. (2000). Biochem. J. 346: 201–208.
Otwinowski, Z. (1993). Oscillation Data Reduction Program. In: Sawye, L., Isaacs, N., and Burley, S., Eds. Proceedings of the CCP4 study weekend: data collection and processing. Warrington, United Kingdom: Daresbury Laboratory. 56–62 (2).
Payan, F. and Qian, M. (2003). J. Protein Chem. 22: 275–284.
Qian, M., Haser, R., Buisson, G., Dúee, E., and Payan, F. (1994). Biochemistry 33: 6284–6294.
Qian, M., Haser, R., and Payan, F. (1993) J.Mol.Biol. 231: 785–799.
Qian, M., Haser, R, and Payan, F. (1995) Protein Sci. 4: 747–755.
Qian, M., Nahoum, V., Bonicel, J., Bischoff, H., Henrissat, B., and Payan, F. (2001). Biochemistry 40: 7700–7709.
Qian, M., Spinelli, S., Driguez, H., and Payan, F. (1997). Protein Sci. 6: 2285–2296.
Ramasubbu, N., Paloth, V., Luo, Y., Brayer, G. D., and Levine, M. J. (1996). Acta Crystallog. D52: 435–446.
Ramasubbu, N., Ragunath, C., and Mishra, P. J. (2003). J. Mol. Biol. 325: 1061–1078.
Read, R. J. (1986). Acta Crystallogr. A42: 140–149.
Robyt, J. F. and French, D. (1970). J. Biol. Chem. 45: 3917–3927.
Roussel, A. and Cambillau, C. (1989). in Silicon graphic geometry partner directory, (Fall, 1989), Silicon Graphics, Mountain View CA., 77–78.
Rydberg, E. H., Li, C., Maurus, R., Overall, C. M., Brayer, G.D., and Withers, S. G. (2002). Biochemistry 41: 4492–4502.
Uitdehaag, J. C. M., Mosi, R., Kalk, K-H., Van der Veen, B. A., Dijkhuizen, L., Withers, S. G., and Dijkstra, B. W. (1999) Nature Structural biology. 6: 432–436.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Zhuo, H., Payan, F. & Qian, M. Crystal Structure of the Pig Pancreatic α-Amylase Complexed with ρ-Nitrophenyl-α-D-Maltoside-Flexibility in the Active Site. J Protein Chem 23, 379–387 (2004). https://doi.org/10.1023/B:JOPC.0000039552.94529.95
Issue Date:
DOI: https://doi.org/10.1023/B:JOPC.0000039552.94529.95