Abstract
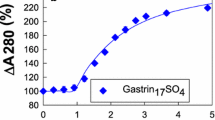
Binding of ferric ions to the hormone glycine-extended gastrin17 is essential for biological activity (Pannequin, J., et al. (2002). J. Biol. Chem. 277: 48602–48609). The aims of the current study were to determine the properties of the complex between recombinant human progastrin6–80 and ferric ions. The stoichiometry and affinity of ferric ion binding were determined by fluorescence spectroscopy. The selectivity of metal ion binding and the stability of the 59Fe(III) progastrin6–80 complex were determined by equilibrium dialysis. The stoichiometry of 2.5 ± 0.1 moles Fe/mole progastrin, and the apparent dissociation constant of 2.2 ± 0.1 μM, were similar to the values previously determined for glycine-extended gastrin17 at pH 4.0. Of the four trivalent and seven divalent metal ions tested, only ferrous and ferric ions bound to progastrin6–80. The ferric ion–progastrin complex was extremely stable, with a half-life of 117 ± 8 days at pH 7.6 and 25°C. We conclude that recombinant human progastrin6–80 selectively binds ferrous and ferric ions with high affinity in a stable 2:1 complex.
Similar content being viewed by others
References
Baldwin, G. S. (1992). Medical Hypotheses 38: 70–74.
Baldwin, G. S., and Shulkes, A. (1998). Gut 42: 581–584.
Baldwin, G. S., Curtain, C. C., and Sawyer, W. H. (2001a). Biochemistry. 40: 10741–10746.
Baldwin, G. S., Hollande, F., Yang, Z., Karelina, Y., Paterson, A., Strang, R., Fourmy, D., Neumann, G., and Shulkes, A. (2001b). J. Biol. Chem. 276: 7791–7796.
Boel, E., Vuust, J., Norris, F., Norris, K., Wind, A., Rehfeld, J. F., and Marcker, K. A. (1983). Proc. Natl. Acad. Sci. U.S.A. 80: 2866–2869.
Edkins, J. S. (1905). Proc. R. Soc. B. 76: 376.
Farkas, E., Csoka, H., Micera, G., and Dessi, A. (1997). J. Inorg. Biochem. 65: 281–286.
Gill, S. C., and von Hippel, P. H. (1989). Analy. Biochem. 182: 319–326.
Gregory, H., Hardy, P. M., Jones, D. S., Kenner, G. W., and Sheppard, R. C. (1964). Nature 204: 931–933.
Huebner, V. D., Jiang, R. L., Lee, T. D., Legesse, K., Walsh, J. H., Shively, J. E., Chew, P., Azumi, T., and Reeve, J. R., Jr. (1991). J. Biol. Chem. 266: 12223–12227.
Palumbo, M., Jaeger, E., Knof, S., Peggion, E., and Wunsch, E. (1980). FEBS Lett. 119: 158–160.
Pannequin, J., Barnham, K. J., Hollande, F., Shulkes, A., Norton, R. S., and Baldwin, G. S. (2002). J. Biol. Chem. 277: 48602–48609.
Peggion, E., Mammi, S., Palumbo, M., Moroder, L., and Wunsch, E. (1983). Biopolymers 22: 2443–2457.
Peggion, E., Mammi, S., Palumbo, M., Moroder, L., and Wunsch, E. (1984). Biopolymers 23: 1225–1240.
Perrin, D. D. (1979). In Stability Constants of Metal-Ion Complexes. Part B. Organic Ligands, Pergamon Press, Oxford, U.K.
Rehfeld, J. F., and Johnsen, A. H. (1994). Eur. J. Biochem. 223: 765–773.
Singh, P., Velasco, M., Given, R., Wargovich, M., Varro, A., and Wang, T. C. (2000). Am. J. Physiol. 278: G390-G399.
Torda, A. E., Baldwin, G. S., and Norton, R. S. (1985). Biochemistry 24: 1720–1727.
Wang, T. C., Koh, T. J., Varro, A., Cahill, R. J., Dangler, C. A., Fox, J. G., and Dockray, G. J. (1996). J. Clin. Invest. 98: 1918–1929.
Winzor, D. J., and Sawyer, W. H. (1995). In Quantitative Characterization of Ligand Binding, Wiley-Liss, New York, pp. 28–41.
Rights and permissions
About this article
Cite this article
Baldwin, G.S. Properties of the Complex Between Recombinant Human Progastrin and Ferric Ions. J Protein Chem 23, 65–70 (2004). https://doi.org/10.1023/B:JOPC.0000016259.59562.6c
Published:
Issue Date:
DOI: https://doi.org/10.1023/B:JOPC.0000016259.59562.6c