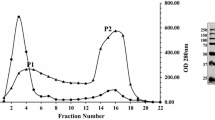
Abstract
A β-glucosidase (BGS) purified from Aspergillus niger cellulase powder (obtained from Sigma, St. Louis, MO, USA) was characterized. Electrophoresis, size exclusion chromatography, and dynamic light scattering indicated that the enzyme is a dimer of approximately 200 kDa. Five of the seven N-glycosylated oligosaccharides attached to BGS were composed of D-mannoses attached to a β(1-4)-N-acetyl-glucosamine-β-(1-4)-fucose-α-(1-6)-N-acetylglucosamine core. The other two were similar, but the cores of these did not have the D-fucose. The enzyme is a retaining glycosidase, and it also has a distinct preference for the β-configuration at the reducing end of cellobiose. BGS is thermostable up to 65°C but is sensitive to freezing and thawing. The extinction coefficient of BGS was found to be 1.8 cm−1mg−1. All substrates assayed resulted in Eadie–Hofstee plots that were curved at high substrate concentrations. TLC of the reaction products showed that the substrates themselves act as acceptors when present at high concentrations. The transglucosidic activity rate is different from the hydrolytic activity rate and this causes the curvature at high substrate concentrations. The enzyme produces gentiobiose when D-glucose is the acceptor. pH optima of the V max{h} with pNPGlc, oNPGlc, and cellobiose were between pH 4 and 4.5, and the K m values decreased at pH values between 3 and 5. Inhibition experiments indicated that the enzyme is specific for glucosyl substrates and suggested that D-gluconolactone is a transition state analog. Studies with cello-oligosaccharides and 3,4-dinitrophenyl-cellobiose showed that BGS is an exo-hydrolase having at least five glucose subsites and that it cleaves from the nonreducing end. The properties of a family 3 β-glucosidase (BG3) sequenced by Dan et al. [Dan, S., Marton, I., Dekel, M., Bravdo, B-A., He, S., Withers, S. G., and Shoseyov, O. (2000) J. Biol. Chem. 275: 4973–4980] was also studied and was shown to have very similar properties to those of BGS. Sequence analysis of a portion of BGS verified that these are the same enzymes.
Similar content being viewed by others
References
Birk, R., Ikan, A., Bravado, B., Braun, S., and Shoseyov, O. (1997). Appl. Biochem. Biotechnol. 66: 25–29.
Capon, B., and Thomson, J. W. (1979). Bioorg. Chem. 8: 147–173.
Dan, S., Marton, I., Dekel, M., Bravdo, B-A., He, S., Withers, S. G., and Shoseyov, O. (2000). J. Biol. Chem. 275: 4973–4980.
Davies, G. J., Wilson, K. S., and Henrissat, B. (1997). Biochemical J. 321: 557–559.
Eadie, G. S. (1942). J. Biol. Chem. 146: 85.
Galas, E., and Romanowska, I. (1997). Acta Microbiologica Polonica 46: 241–252.
Gueguen, Y., Chemardin, P., Pien, S., Arnaud, A., and Galzy, P. (1997). J. Biotechnol. 55: 151–156.
Gupte, A., and Madamwar, D. (1997) Biotechnol. Prog. 13: 166–169.
Henrissat, B. (1991). Biochemical J. 280: 309–316.
Hofstee, B. H. J. (1952). J. Biol. Chem. 199: 357.
Hoh, Y. K., Yeoh, H-H., and Tan, T. K. (1992). Appl. Microbiol. Biotechnol. 37: 590–593.
Kruger, N. J. (1994). Methods Mol. Biol. 32: 9–15.
Kuriyama, K. (1995). Biosci. Biotechnol. Biochem. 59: 1142–1143.
Leah, R., Kigel, J., Svendsen, I., and Mundy, J. (1995). J. Biol. Chem. 270: 15789–15797.
Mandels, M. (1985). Biochem Soc. Trans. 13: 414.
Martino, A., Pifferi, P. G., and Spagna, G. (1994). J. Chem. Technol. Biotechnol. 60: 247–252.
Mikhaylova, M., Wiederschain, G., Mikhaylov, V., and Aerts, J. M. F. G. (1996). Biochim. Biophys. Acta 1317: 71–79.
Ohnishi, M., Okada, G., and Yazaki, T. (1998). Carbohydr. Res. 308: 201–205.
Panchal, T., and Wodzinski, R. J. (1998). Prep. Biochem. Biotechnol. 28: 201–217.
Parke, S. A., Birch, G. G., MacDougall, D. B., and Stevens, D. A. (1997). Chemical Senses 22: 53–65.
Rouvinen, J., Bergfors, T., Teeri, T., Knowles, J. K. C., and Jones, T. A. (1990). Science 249: 380–385.
Rudick, M. J., and Elbein, A. D. (1973). J. Biol. Chem. 248: 6506–6513.
Shakin-Eshleman, S. H., Spitsalnik, S. L., and Kasturi, L. (1996). J. Biol. Chem. 271: 6363–6366.
Skory, C., Freer, S. N., and Bothast, R. J. (1996). Current Genetics 30: 417–422.
Wood, T. M. (1992). Biochem. Soc. Trans. 20: 46–53.
Woodward, J., and Wiseman, A. (1982). Enzyme and Microbial Technology 4: 73–79.
Yazaki, T., Ohnishi, M., Rokushika, S., and Okada, G. (1997). Carbohydr. Res. 298: 51–57.
Rights and permissions
About this article
Cite this article
Seidle, H.F., Marten, I., Shoseyov, O. et al. Physical and Kinetic Properties of the Family 3 β-Glucosidase from Aspergillus niger Which Is Important for Cellulose Breakdown. J Protein Chem 23, 11–23 (2004). https://doi.org/10.1023/B:JOPC.0000016254.58189.2a
Published:
Issue Date:
DOI: https://doi.org/10.1023/B:JOPC.0000016254.58189.2a