Abstract
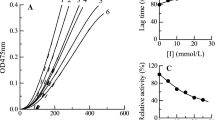
Cetylpyridinium chloride (CPC) was found to inactivate tyrosinase from mushroom (Agaricus bisporus). CPC can bind to the enzyme molecule and induce the enzyme conformation changes. The fluorescence intensity (at 338.4 nm) of the enzyme decreased distinctly with increasing CPC concentrations, and a new little fluorescence emission peak appeared near 372 nm. The inactivation of the enzyme by CPC had first been studied by using the kinetic method of the substrate reaction described by Tsou. The results showed that the enzyme was inactivated by a complex mechanism that had not been previously identified. The enzyme first quickly binds with CPC reversibly and then undergoes a slow irreversible inactivation. The inactivation reaction is a single molecule reaction and the apparent inactivation rate constant is a saturated trend being independent of CPC concentration if the concentration is sufficiently high. The micro rate constants of inactivation and the association constant were determined.
Similar content being viewed by others
REFERENCES
Asada, N., Fukumitsu, T., Fujimoto, K., and Masuda, K. I. (1993). Insert. Biochem. Mol. Biol. 23: 515–520.
Chen, Q. X., Zhang, Z., Zhou, X. W., and Zhuang, Z. L. (2000a). Int. J. Biochem. Cell Biol. 32: 717–723.
Chen, Q. X., Zheng, W. Z., Lin, J. Y., Cai, Z. T., and Zhou, H. (2000b). Biochemistry (Moscow) 65: 1105–1110.
Espín, J. C., and Wichers, H. J. (1999). J. Agric. Food Chem. 47: 2638–2644.
Espín, J. C., Tudela, J., and García-Cánovas, F. (1998). Anal. Biochem. 259: 118–126.
Espín, J. C., and Wichers, H. J. (1999). J. Agric. Food Chem. 47: 3518–3525.
Hall, M., Scott, T., Sugumaran, M., Soderhall, K., and Law, J. H. (1995). Proc. Natl. Acad. Sci. USA. 92: 7764–7768.
Jiménez, M., and García-Carmona, F. (1996). Phytochemistry 42: 1503–1509.
Jiménez, M., Chazarra, S., Escribano, J., Cabanes, J., and Garcia-Carmína, F. (2001). J. Agric. Food Chem. 49: 4060–4063.
Kenten, R. H. (1957). Biochem. J. 33: 193–200.
Moore, B. M., and Flurkey, W. H. (1990). J. Biol. Chem. 265: 4982–4988.
Martínez, M. V., and Whitaker, J. R. (1995). Trends Food Sci. Technol. 6: 195–200.
Nellaiappan, K., and Sugumaran, M. (1996). Comp. Biochem. Physiol. 113B: 163–168.
Prota, G. (1988). Med. Res. Rev. 8: 525–556.
Rodríguez-López, J. N., Fenoll, L. G., and García-Cánovas, F. (2000). Biochemistry 39: 10497–10506.
Sánchez-Ferrer, A., Rodríguez-López, J. N., García-Cánovas, F., and García-Carmona, F. (1995). Biochem. Biophys. Acta 1247: 1–11.
Sugumaran, M., and Nellaiappan, K. (1991). Biochem. Biophys. Res. Commun. 176: 1371–1376.
Soler-Rivas, C., Arpin, N., Olivier, J. M., and Wichers, H. J. (1997). Mycol. Res. 101: 375–382.
Tsou, C. L. (1988). Adv. Enzymol. Relat. Areas Mol. Biol. 61: 381–436.
Van Leeuwen, J., and Wichers, H. (1999). Mycol. Res. 103: 413–418.
Witaker, J. R. (1995). Food Enzymes Structure and Mechanism (Wong, D. W. S., ed.), Chapman Hall, New York, pp. 271–307.
Yamaguchi, M., Hwang, P. M., and Campbell, J. D. (1970). Can. J. Biochem. 48: 198–202.
Zawistowski, J., Biliaderis, C. G., and Eskin, N. A. M. (1991). Oxidative Enzyme in Foods (Robinson, D. S., and Eskin, N. A. M., eds.), Elsevier Science, London, pp. 217–273.
Zhou, X. W., Zhuang, Z. L., and Chen, Q. X. (1999). J. Prot. Chem. 32: 735–740.
Zhang, R. Q., Chen, Q. X., Xiao, R., Xie, L. P., Zeng, X. G., and Zhou, H. M. (2001). Biochim. Biophys. Acta 1545: 6–12.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, QX., Huang, H. & Kubo, I. Inactivation Kinetics of Mushroom Tyrosinase by Cetylpyridinium Chloride. J Protein Chem 22, 481–487 (2003). https://doi.org/10.1023/B:JOPC.0000005464.36961.9c
Published:
Issue Date:
DOI: https://doi.org/10.1023/B:JOPC.0000005464.36961.9c