Abstract
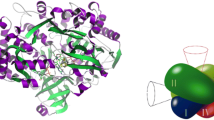
Ethanol oxidation by nicotinoprotein alcohol dehydrogenase (np-ADH) from the bacterium Amycolatopsis methanolica is inhibited by trans-4-(N,N-dimethylamino)-cinnamaldehyde through direct binding to the catalytic zinc ion in a substrate-like geometry. This binding is accompanied by a characteristic red shift of the aldehyde absorbance from 398 nm to 467 nm. Np-ADH is structurally related to mammalian ADH class I, and a model of np-ADH shows how the cinnamaldehyde derivative can be accommodated in the active site of the nicotinoprotein, correlating the structural and enzymological data.
Similar content being viewed by others
REFERENCES
Cedergren-Zeppezauer, E., Samama, J.-P., and Eklund, H. (1982). Crystal structure determinations of coenzyme analogue and substrate complexes of liver alcohol dehydrogenase: Binding of 1,4,5,6-tetrahydronicotinamide adenine dinucleotide and trans-4-(N,N-dimethylamino)cinnamaldehyde to the enzyme. Biochemistry 21: 4895–4908.
Cornish-Bowden, A. (1995). Analysis of enzyme kinetic data, p. 198, Oxford University press, Oxford.
Dahl, K. H., and Dunn, M. F. (1984). Reaction of 4-trans-(N,Ndimethylamino) cinnamaldehyde with the liver alcohol dehydrogenase-oxidized nicotinamide adenine dinucleotide complex. Biochemistry 23: 4094–4100.
Dietrich, H., Maret, W., Wallén, L., and Zeppezauer, M. (1979). Active-site-specific reconstituted cobalt(II) horse-liver alcohol dehydrogenase. Changes of the spectra of the substrate trans-4-(N,N-dimethylamino)-cinnamaldehyde and of the catalytic cobalt ion upon ternary complex formation with NADH and 1,4,5,6-tetrahydronicotinamide-adenine dinucleotide. Eur J Biochem. 100: 267–270.
Dunn, M. F., and Bernhard, S. A. (1971). Rapid kinetic evidence for adduct formation between the substrate analog p-nitroso-N,Ndimethylaniline and reduced nicotinamide-adenine dinucleotide during enzymic reduction. Biochemistry 10: 4569–4575.
Dunn, M. F., and Hutchinson, J. S. (1973). Roles of zinc ion and reduced coenzyme in the formation of a transient chemical intermediate during the equine liver alcohol dehydrogenase catalyzed reduction of an aromatic aldehyde. Biochemistry 12: 4882–4892.
Dunn, M. F., Dietrich, H., MacGibbon, A. K. H., Koerber, S. C., and Zeppezauer, M. (1982). Investigation of intermediates and transition states in the catalytic mechanisms of active site substituted cobalt(II), nickel(II), zinc(II), and cadmium(II) horse liver alcohol dehydrogenase. Biochemistry 21: 354–363.
Eklund, H., Müller-Wille, P., Horjales, E., Futer, O., Holmquist, B., Vallee, B.L., Höög, J.-O., Kaiser, R., and Jörnvall, H. (1990). Comparison of three classes of human liver alcohol dehydrogenase. Emphasis on different substrate-binding pockets. Eur. J. Biochem. 193: 303–310.
Jörnvall, H., Nordling, E., and Persson, B. (2003). Multiplicity of eukaryotic ADH and other MDR forms. Chem.-Biol. Interact. 143–144: 255–261.
Laemmli, U. K. (1970). Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685.
Norin, A., Piersma, S. R., Duine, J. A., and Jörnvall, H. (2003). Nicotinoprotein (NAD+-containing) alcohol dehydrogenase: Structural relationships and functional interpretations. Cell. Mol. Life Sci. 60: in press.
Piersma, S. R., Visser, A. J. W. G., de Vries, S., and Duine, J. A. (1998). Optical spectroscopy of nicotinoprotein alcohol dehydrogenase from Amycolatopsis methanolica: A comparison with horse liver alcohol dehydrogenase and UDP-galactose epimerase. Biochemistry 37: 3068–3077.
Ramaswamy, S., Eklund, H., and Plapp, B. V. (1994). Structures of horse liver alcohol dehydrogenase complexed with NAD+ and substituted benzyl alcohols. Biochemistry 33: 5230–5237.
Schneider-Bernlöhr, H., Formicka-Kozlowska, G., Bühler, R., von Wartburg, J.-P., and Zeppezauer, M. (1988). Active-site-specific zinc-depleted and reconstituted cobalt(II) human-liver alcohol dehydrogenase. Preparation, characterization and complexation with NADH and trans-4-(N,N-dimethylamino)-cinnamaldehyde. Eur. J. Biochem. 173: 275–280.
Stone, C. L., Bosron, W. F., and Dunn, M. F. (1993). Amino acid substitutions at position 47 of human β1β1 and β2β2 alcohol dehydrogenases affect hydride transfer and coenzyme dissociation rate constants. J. Biol. Chem. 268: 892–899.
van Ophem, P. W., van Beeumen, J., and Duine, J. A. (1993). Nicotinoprotein 'NAD(P)-containing' alcohol/aldehyde oxidoreductases. Purification and characterization of a novel type from Amycolatopsis methanolica. Eur. J. Biochem. 156: 819–826.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Piersma, S.R., Norin, A., de Vries, S. et al. Inhibition of Nicotinoprotein (NAD+-Containing) Alcohol Dehydrogenase by trans-4-(N,N-Dimethylamino)-Cinnamaldehyde Binding to the Active Site. J Protein Chem 22, 457–461 (2003). https://doi.org/10.1023/B:JOPC.0000005461.53788.ee
Published:
Issue Date:
DOI: https://doi.org/10.1023/B:JOPC.0000005461.53788.ee