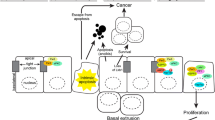
Abstract
Tight junctions (TJs), the most apical components of the cell–cell junctional complexes, play a crucial role in the establishment and maintenance of cell polarity within tissues. In secretory glandular tissues, such as the mammary gland, TJs are crucial for separating apical and basolateral domains. TJs also create the variable barrier regulating paracellular movement of molecules through epithelial sheets, thereby maintaining tissue homeostasis. Recent advances reveal that TJs exist as macromolecular complexes comprised of several types of membrane proteins, cytoskeletal proteins, and signaling molecules. Many of these components are regulated during mammary gland development and pregnancy cycles, and several have received much attention as possible “tumor suppressors” during progression to breast cancer.
Similar content being viewed by others
REFERENCES
M. G. Farquhar and G. E. Palade (1963). Junctional complexes in various epithelia. J. Cell Biol. 17: 375–412.
E. E. Schneeberger and R. D. Lynch (1992). Structure, function, and regulation of cellular tight junctions. Am. J. Physiol. 262: L647-L661.
S. Tsukita, M. Furuse, and M. Itoh (2001). Multifunctional strands in tight junctions. Nat. Rev. Mol. Cell Biol. 2: 285–293.
B. M. Gumbiner (1993). Breaking through the tight junction barrier. J. Cell Biol. 123: 1631–1633.
L. A. Staehelin (1973). Further observations on the fine structure of freeze-cleaved tight junctions. J. Cell Sci. 13: 763–786.
D. C. Baumgart and A. U. Dignass (2002). Intestinal barrier function. Curr. Opin. Clin. Nutr. Metab. Care 5: 685–694.
E. S. Barton, J. C. Forrest, J. L. Connolly, J. D. Chappell, Y. Liu, F. J. Schnell, A. Nusrat, C. A. Parkos, and T. S. Dermody (2001). Junction adhesion molecule is a receptor for reovirus. Cell 104: 441–451.
M. R. Amieva, R. Vogelmann, A. Covacci, L. S. Tompkins, W. J. Nelson, and S. Falkow (2003). Disruption of the epithelial apical-junctional complex by Helicobacter pylori CagA. Science 300: 1430–1434.
J. M. Staddon and L. L. Rubin (1996). Cell adhesion, cell junctions and the blood-brain barrier. Curr. Opin. Neurobiol. 6: 622–627.
J. A. Holash, S. I. Harik, G. Perry, and P. A. Stewart (1993). Barrier properties of testis microvessels. Proc. Natl. Acad. Sci. U.S.A. 90: 11069–11073.
J. M. Scherrmann (2002). Drug delivery to brain via the blood-brain barrier. Vascul. Pharmacol. 38: 349–354.
K. R. Spring (1998). Routes and mechanism of fluid transport by epithelia. Annu. Rev. Physiol. 60: 105–119.
D. Bilder (2001). PDZ proteins and polarity: functions from the fly. Trends Genet. 17: 511–519.
U. Tepass, G. Tanentzapf, R. Ward, and R. Fehon (2001). Epithelial cell polarity and cell junctions in Drosophila. Annu. Rev. Genet. 35: 747–784.
T. Nakamura, J. Blechman, S. Tada, T. Rozovskaia, T. Itoyama, F. Bullrich, A. Mazo, C. M. Croce, B. Geiger, and E. Canaani (2000). huASH1 protein, a putative transcription factor encoded by a human homologue of the Drosophila ash1 gene, localizes to both nuclei and cell-cell tight junctions. Proc. Natl. Acad. Sci. U.S.A. 97: 7284–7289.
M. S. Balda and K. Matter (2000). The tight junction protein ZO-1 and an interacting transcription factor regulate ErbB-2 expression. EMBO. J. 19: 2024–2033.
M. S. Balda, M. D. Garrett, and K. Matter (2003). The ZO-1-associated Y-box factor ZONAB regulates epithelial cell proliferation and cell density. J. Cell Biol. 160: 423–432.
M. Furuse, T. Hirase, M. Itoh, A. Nagafuchi, S. Yonemura, and S. Tsukita (1993). Occludin: A novel integral membrane protein localizing at tight junctions. J. Cell Biol. 123: 1777–1788.
V. Wong (1997). Phosphorylation of occludin correlates with occludin localization and function at the tight junction. Am. J. Physiol. 273: C1859-C1867.
M. Saitou, K. Fujimoto, Y. Doi, M. Itoh, T. Fujimoto, M. Furuse, H. Takano, T. Noda, and S. Tsukita (1998). Occludin-deficient embryonic stem cells can differentiate into polarized epithelial cells bearing tight junctions. J. Cell Biol. 141: 397–408.
M. Furuse, K. Fujita, T. Hiiragi, K. Fujimoto, and S. Tsukita (1998). Claudin-1 and-2: Novel integral membrane proteins localizing at tight junctions with no sequence similarity to occludin. J. Cell Biol. 141: 1539–1550.
M. Furuse, H. Sasaki, K. Fujimoto, and S. Tsukita (1998). A single gene product, claudin-1 or-2, reconstitutes tight junction strands and recruits occludin in fibroblasts. J. Cell Biol. 143: 391–401.
Y. Kiuchi-Saishin, S. Gotoh, M. Furuse, A. Takasuga, Y. Tano, and S. Tsukita (2002). Differential expression patterns of claudins, tight junction membrane proteins, in mouse nephron segments. J. Am. Soc. Nephrol. 13: 875–886.
D. B. Simon, Y. Lu, K. A. Choate, H. Velazquez, E. Al-Sabban, M. Praga, G. Casari, A. Bettinelli, G. Colussi, J. Rodriguez-Soriano, D. McCredie, D. Milford, S. Sanjad, and R. P. Lifton (1999). Paracellin-1, a renal tight junction protein required for paracellular Mg2+ resorption. Science 285: 103–106.
E. R. Wilcox, Q. L. Burton, S. Naz, S. Riazuddin, T. N. Smith, B. Ploplis, I. Belyantseva, T. Ben-Yosef, N. A. Liburd, R. J. Morell, B. Kachar, D. K. Wu, A. J. Griffith, and T. B. Friedman (2001). Mutations in the gene encoding tight junction claudin-14 cause autosomal recessive deafness DFNB29. Cell 104: 165–172.
K. Morita, H. Sasaki, M. Furuse, and S. Tsukita (1999). Endothelial claudin: Claudin-5/TMVCF constitutes tight junction strands in endothelial cells. J. Cell Biol. 147: 185–194.
T. Nitta, M. Hata, S. Gotoh, Y. Seo, H. Sasaki, N. Hashimoto, M. Furuse, and S. Tsukita (2003). Size-selective loosening of the blood-brain barrier in claudin-5-deficient mice. J. Cell Biol. 161: 653–660.
A. Gow, C. M. Southwood, J. S. Li, M. Pariali, G. P. Riordan, S. E. Brodie, J. Danias, J. M. Bronstein, B. Kachar, and R. A. Lazzarini (1999). CNS myelin and Sertoli cell tight junction strands are absent in Osp/claudin-11 null mice. Cell 99: 649–659.
M. Furuse, M. Hata, K. Furuse, Y. Yoshida, A. Haratake, Y. Sugitani, T. Noda, A. Kubo, and S. Tsukita (2002). Claudin-based tight junctions are crucial for the mammalian epidermal barrier: A lesson from claudin-1-deficient mice. J. Cell Biol. 156: 1099–1111.
J. M. Diamond (1978). Channels in epithelial cell membranes and junctions. Fed. Proc. 37: 2639–2643.
I. Martin-Padura, S. Lostaglio, M. Schneemann, L. Williams, M. Romano, P. Fruscella, C. Panzeri, A. Stoppacciaro, L. Ruco, A. Villa, D. Simmons, and E. Dejana (1998). Junctional adhesion molecule, a novel member of the immunoglobulin superfamily that distributes at intercellular junctions and modulates monocyte transmigration. J. Cell Biol. 142: 117–127.
M. Itoh, H. Sasaki, M. Furuse, H. Ozaki, T. Kita, and S. Tsukita (2001). Junctional adhesion molecule (JAM) binds to PAR-3: A possible mechanism for the recruitment of PAR-3 to tight junctions. J. Cell Biol. 154: 491–497.
K. Ebnet, A. Suzuki, Y. Horikoshi, T. Hirose, Z. Meyer, M. K. Brickwedde, S. Ohno, and D. Vestweber (2001). The cell polarity protein ASIP/PAR-3 directly associates with junctional adhesion molecule (JAM). EMBO. J. 20: 3738–3748.
A. Suzuki, C. Ishiyama, K. Hashiba, M. Shimizu, K. Ebnet, and S. Ohno (2002). aPKC kinase activity is required for the asymmetric differentiation of the premature junctional complex during epithelial cell polarization. J. Cell Sci. 115: 3565–3573.
L. B. Spiryda and D. R. Colman (1998). Protein zero, a myelin IgCAM, induces physiologically operative tight junctions in nonadhesive carcinoma cells. J. Neurosci. Res. 54: 282–288.
S. K. Tiwari-Woodruff, A. G. Buznikov, T. Q. Vu, P. E. Micevych, K. Chen, H. I. Kornblum, and J. M. Bronstein (2001). OSP/claudin-11 forms a complex with a novel member of the tetraspanin super family and beta1 integrin and regulates proliferation and migration of oligodendrocytes. J. Cell Biol. 153: 295–305.
C. J. Cohen, J. T. Shieh, R. J. Pickles, T. Okegawa, J. T. Hsieh, and J. M. Bergelson (2001). The coxsackievirus and adenovirus receptor is a transmembrane component of the tight junction. Proc. Natl. Acad. Sci. U.S.A. 98: 15191–15196.
B. R. Stevenson, J. D. Siliciano, M. S. Mooseker, and D. A. Goodenough (1986). Identification of ZO-1: A high molecular weight polypeptide associated with the tight junction (zonula occludens) in a variety of epithelia. J. Cell Biol. 103: 755–766.
B. Gumbiner, T. Lowenkopf, and D. Apatira (1991). Identification of a 160-kDa polypeptide that binds to the tight junction protein ZO-1. Proc. Natl. Acad. Sci. U.S.A. 88: 3460–3464.
L. A. Jesaitis and D. A. Goodenough (1994). Molecular characterization and tissue distribution of ZO-2, a tight junction protein homologous to ZO-1 and the Drosophila discs-large tumor suppressor protein. J. Cell Biol. 124: 949–961.
J. Haskins, L. Gu, E. S. Wittchen, J. Hibbard, and B. R. Stevenson (1998). ZO-3, a novel member of the MAGUK protein family found at the tight junction, interacts with ZO-1 and occludin. J. Cell Biol. 141: 199–208.
M. Itoh, A. Nagafuchi, S. Yonemura, T. Kitani-Yasuda, and S. Tsukita (1993). The 220-kD protein colocalizing with cadherins in non-epithelial cells is identical to ZO-1, a tight junction-associated protein in epithelial cells: cDNA cloning and immunoelectron microscopy. J. Cell Biol. 121: 491–502.
E. Willott, M. S. Balda, A. S. Fanning, B. Jameson, C. Van Itallie, and J. M. Anderson (1993). The tight junction protein ZO-1 is homologous to the Drosophila discs-large tumor suppressor protein of septate junctions. Proc. Natl. Acad. Sci. U.S.A. 90: 7834–7838.
M. B. Kennedy (1995). Origin of PDZ (DHR, GLGF) domains. Trends Biochem. Sci. 20: 350.
M. Sheng and C. Sala (2001). PDZ domains and the organization of supramolecular complexes. Annu. Rev. Neurosci. 24: 1–29.
Z. Songyang, A. S. Fanning, C. Fu, J. Xu, S. M. Marfatia, A. H. Chishti, A. Crompton, A. C. Chan, J. M. Anderson, and L. C. Cantley (1997). Recognition of unique carboxyl-terminal motifs by distinct PDZ domains. Science 275: 73–77.
M. Itoh, M. Furuse, K. Morita, K. Kubota, M. Saitou, and S. Tsukita (1999). Direct binding of three tight junction-associated MAGUKs, ZO-1, ZO-2, and ZO-3, with the COOH termini of claudins. J. Cell Biol. 147: 1351–1363.
M. Furuse, M. Itoh, T. Hirase, A. Nagafuchi, S. Yonemura, and S. Tsukita (1994). Direct association of occludin with ZO-1 and its possible involvement in the localization of occludin at tight junctions. J. Cell Biol. 127: 1617–1626.
A. S. Fanning, B. J. Jameson, L. A. Jesaitis, and J. M. Anderson (1998). The tight junction protein ZO-1 establishes a link between the transmembrane protein occludin and the actin cytoskeleton. J. Biol. Chem. 273: 29745–29753.
M. Itoh, K. Morita, and S. Tsukita (1999). Characterization of ZO-2 as a MAGUK family member associated with tight as well as adherens junctions with a binding affinity to occludin and alpha catenin. J. Biol. Chem. 274: 5981–5986.
M. Itoh, A. Nagafuchi, S. Moroi, and S. Tsukita (1997). Involvement of ZO-1 in cadherin-based cell adhesion through its direct binding to alpha catenin and actin filaments. J. Cell Biol. 138: 181–192.
E. S. Wittchen, J. Haskins, and B. R. Stevenson (2000). Exogenous expression of the amino-terminal half of the tight junction protein ZO-3 perturbs junctional complex assembly. J. Cell Biol. 151: 825–836.
B. Etemad-Moghadam, S. Guo, and K. J. Kemphues (1995). Asymmetrically distributed PAR-3 protein contributes to cell polarity and spindle alignment in early C. elegans embryos. Cell 83: 743–752.
K. Kemphues (2000). PARsing embryonic polarity. Cell 101: 345–348.
C. Q. Doe (2001). Cell polarity: The PARty expands. Nat. Cell Biol. 3: E7-E9.
Y. Izumi, T. Hirose, Y. Tamai, S. Hirai, Y. Nagashima, T. Fujimoto, Y. Tabuse, K. J. Kemphues, and S. Ohno (1998). An atypical PKC directly associates and colocalizes at the epithelial tight junction with ASIP, a mammalian homologue of Caenorhabditis elegans polarity protein PAR-3. J. Cell Biol. 143: 95–106.
R. G. Qiu, A. Abo, and G. Steven Martin (2000). A human homolog of the C. elegans polarity determinant Par-6 links Rac and Cdc42 to PKCzeta signaling and cell transformation. Curr. Biol. 10: 697–707.
G. Joberty, C. Petersen, L. Gao, and I. G. Macara (2000). The cell-polarity protein Par6 links Par3 and atypical protein kinase C to Cdc42. Nat. Cell Biol. 2: 531–539.
Y. Hamazaki, M. Itoh, H. Sasaki, M. Furuse, and S. Tsukita (2002). Multi-PDZ domain protein 1 (MUPP1) is concentrated at tight junctions through its possible interaction with claudin-1 and junctional adhesion molecule. J. Biol. Chem. 277: 455–461.
M. H. Roh, O. Makarova, C. J. Liu, K. Shin, S. Lee, S. Laurinec, M. Goyal, R. Wiggins, and B. Margolis (2002). The Maguk protein, Pals1, functions as an adapter, linking mammalian homologues of Crumbs and Discs Lost. J. Cell Biol. 157: 161–172.
X. Wu, K. Hepner, S. Castelino-Prabhu, D. Do, M. B. Kaye, X. J. Yuan, J. Wood, C. Ross, C. L. Sawyers, and Y. E. Whang (2000). Evidence for regulation of the PTEN tumor suppressor by a membrane-localized multi-PDZ domain containing scaffold protein MAGI-2. Proc. Natl. Acad. Sci. U.S.A. 97: 4233–4238.
Y. Wu, D. Dowbenko, S. Spencer, R. Laura, J. Lee, Q. Gu, and L. A. Lasky (2000). Interaction of the tumor suppressor PTEN/MMAC with a PDZ domain of MAGI3, a novel membrane-associated guanylate kinase. J. Biol. Chem. 275: 21477–21485.
N. Ide, Y. Hata, H. Nishioka, K. Hirao, I. Yao, M. Deguchi, A. Mizoguchi, H. Nishimori, T. Tokino, Y. Nakamura, and Y. Takai (1999). Localization of membrane-associated guanylate kinase (MAGI)-1/BAI-associated protein (BAP) 1 at tight junctions of epithelial cells. Oncogene 18: 7810–7815.
S. Citi, H. Sabanay, R. Jakes, B. Geiger, and J. Kendrick-Jones (1988). Cingulin, a new peripheral component of tight junctions. Nature 333: 272–276.
Y. Zhong, T. Saitoh, T. Minase, N. Sawada, K. Enomoto, and M. Mori (1993). Monoclonal antibody 7H6 reacts with a novel tight junction-associated protein distinct from ZO-1, cingulin and ZO-2. J. Cell Biol. 120: 477–483.
B. H. Keon, S. Schafer, C. Kuhn, C. Grund, and W. W. Franke (1996). Symplekin, a novel type of tight junction plaque protein. J. Cell Biol. 134: 1003–1018.
E. Weber, G. Berta, A. Tousson, St. P. John, and M. W. Green, U. Gopalokrishnan, T. Jilling, E. J. Sorscher, T. S. Elton, and D. R. Abrahamson (1994). Expression and polarized targeting of a rab3 isoform in epithelial cells. J. Cell Biol. 125: 583–594.
M. Nishimura, M. Kakizaki, Y. Ono, K. Morimoto, M. Takeuchi, Y. Inoue, T. Imai, and Y. Takai (2002). JEAP, a novel component of tight junctions in exocrine cells. J. Biol. Chem. 277: 5583–5587.
H. Kawabe, H. Nakanishi, M. Asada, A. Fukuhara, K. Morimoto, M. Takeuchi, Y. Takai (2001). Pilt, a novel peripheral membrane protein at tight junctions in epithelial cells. J. Biol. Chem. 276: 48350–48355.
A. Suzuki, T. Yamanaka, T. Hirose, N. Manabe, K. Mizuno, M. Shimizu, K. Akimoto, Y. Izumi, T. Ohnishi, and S. Ohno (2001). Atypical protein kinase C is involved in the evolutionarily conserved par protein complex and plays a critical role in establishing epithelia-specific junctional structures. J. Cell Biol. 152: 1183–1196.
B. M. Denker, C. Saha, S. Khawaja, and S. K. Nigam (1996). Involvement of a heterotrimeric G protein alpha subunit in tight junction biogenesis. J. Biol. Chem. 271: 25750–25753.
C. Klingler, U. Kniesel, S. D. Bamforth, H. Wolburg, B. Engelhardt, and W. Risau (2000). Disruption of epithelial tight junctions is prevented by cyclic nucleotide-dependent protein kinase inhibitors. Histochem. Cell Biol. 113: 349–361.
J. M. Mullin, K. V. Laughlin, N. Ginanni, C. W. Marano, H. M. Clarke, and A. Peralta Soler (2000). Increased tight junction permeability can result from protein kinase C activation/translocation and act as a tumor promotional event in epithelial cancers. Ann. N.Y. Acad. Sci. 915: 231–236.
G. Morgan and F. B. Wooding (1982). A freeze-fracture study of tight junction structure in sheep mammary gland epithelium during pregnancy and lactation. J. Dairy Res. 49: 1–11.
K. Stelwagen, D. C. van Espen, G. A. Verkerk, H. A. McFadden, and V. C. Farr (1998). Elevated plasma cortisol reduces permeability of mammary tight junctions in the lactating bovine mammary epithelium. J. Endocrinol. 159: 173–178.
D. A. Nguyen and M. C. Neville (1998). Tight junction regulation in the mammary gland. J. Mam. Gland Biol. Neoplasia 3: 233–246.
D. A. Nguyen, A. F. Parlow, and M. C. Neville (2001). Hormonal regulation of tight junction closure in the mouse mammary epithelium during the transition from pregnancy to lactation. J. Endocrinol. 170: 347–356.
N. M. Rubenstein, Y. Guan, P. L. Woo, and G. L. Firestone (2003). Glucocorticoid down-regulation of RhoA is required for the steroid-induced organization of the junctional complex and tight junction formation in rat mammary epithelial tumor cells. J. Biol. Chem. 278: 10353–10360.
P. L. Woo, D. Ching, Y. Guan, and G. L. Firestone (1999). Requirement for Ras and phosphatidylinositol 3-kinase signaling uncouples the glucocorticoid-induced junctional organization and transepithelial electrical resistance in mammary tumor cells. J. Biol. Chem. 274: 32818–32828.
K. L. Singer, B. R. Stevenson, P. L. Woo, and G. L. Firestone (1994). Relationship of serine/threonine phosphorylation/dephosphorylation signaling to glucocorticoid regulation of tight junction permeability and ZO-1 distribution in nontransformed mammary epithelial cells. J. Biol. Chem. 269: 16108–16115.
K. Stelwagen, H. A. McFadden, and J. Demmer (1999). Prolactin, alone or in combination with glucocorticoids, enhances tight junction formation and expression of the tight junction protein occludin in mammary cells. Mol. Cell Endocrinol. 156: 55–61.
M. Mareel and A. Leroy (2003). Clinical, cellular, and molecular aspects of cancer invasion. Physiol. Rev. 83: 337–376.
W. Birchmeier (1995). E-cadherin as a tumor (invasion) suppressor gene. Bioessays 17: 97–99.
K. B. Hoover, S. Y. Liao, and P. J. Bryant (1998). Loss of the tight junction MAGUK ZO-1 in breast cancer: Relationship to glandular differentiation and loss of heterozygosity. Am. J. Pathol. 153: 1767–1773.
L. Mauro, M. Bartucci, C. Morelli, S. Ando, and E. Surmacz (2001). IGF-I receptor-induced cell-cell adhesion of MCF-7 breast cancer cells requires the expression of junction protein ZO-1. J. Biol. Chem. 276: 39892–39897.
A. Chlenski, K. V. Ketels, G. I. Korovaitseva, M. S. Talamonti, R. Oyasu, D. G. Scarpelli (2000). Organization and expression of the human zo-2 gene (tjp-2) in normal and neoplastic tissues. Biochim. Biophys. Acta. 1493: 319–324.
B. A. Glaunsinger, R. S. Weiss, S. S. Lee, and R. Javier (2001). Link of the unique oncogenic properties of adenovirus type 9 E4-ORF1 to a select interaction with the candidate tumor suppressor protein ZO-2. EMBO J. 20: 5578–5586.
S. L. Kominsky, P. Argani, D. Korz, E. Evron, V. Raman, E. Garrett, A. Rein, G. Sauter, O. P. Kallioniemi, and S. Sukumar (2003). Loss of the tight junction protein claudin-7 correlates with histological grade in both ductal carcinoma in situ and invasive ductal carcinoma of the breast. Oncogene 22: 2021–2033.
T. Hoevel, R. Macek, O. Mundigl, K. Swisshelm, and M. Kubbies (2002). Expression and targeting of the tight junction protein CLDN1 in CLDN1-negative human breast tumor cells. J. Cell Physiol. 191: 60–68.
K. Swisshelm, A. Machl, S. Planitzer, R. Robertson, M. Kubbies, and S. Hosier (1999). SEMP1, a senescence-associated cDNA isolated from human mammary epithelial cells, is a member of an epithelial membrane protein superfamily. Gene 226: 285–295.
L. B. Rangel, R. Agarwal, T. D'Souza, E. S. Pizer, P. L. Alo, W. D. Lancaster, L. Gregoire, D. R. Schwartz, K. R. Cho, and P. J. Morin (2003). Tight junction proteins claudin-3 and claudin-4 are frequently overexpressed in ovarian cancer but not in ovarian cystadenomas. Clin. Cancer Res. 9: 2567–2575.
C. D. Hough, C. A. Sherman-Baust, E. S. Pizer, F. J. Montz, D. D. Im, N. B. Rosenshein, K. R. Cho, G. J. Riggins, and P. J. Morin (2000). Large-scale serial analysis of gene expression reveals genes differentially expressed in ovarian cancer. Cancer Res. 60: 6281–6287.
N. Miwa, M. Furuse, S. Tsukita, N. Niikawa, Y. Nakamura, Y. Furukawa (2000). Involvement of claudin-1 in the beta-catenin/Tcf signaling pathway and its frequent upregulation in human colorectal cancers. Oncol. Res. 12: 469–476.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Itoh, M., Bissell, M.J. The Organization of Tight Junctions in Epithelia: Implications for Mammary Gland Biology and Breast Tumorigenesis. J Mammary Gland Biol Neoplasia 8, 449–462 (2003). https://doi.org/10.1023/B:JOMG.0000017431.45314.07
Issue Date:
DOI: https://doi.org/10.1023/B:JOMG.0000017431.45314.07