Abstract
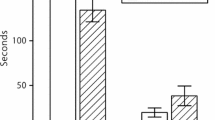
We investigated whether corn plants treated with jasmonic acid (JA) increases the ability of the parasitic wasp, Cotesia kariyai, to find and control the common armyworm (Mythimna separata) under laboratory conditions. The rank order of attractiveness increased from intact plants treated with distilled water (DW) (2 d), JA-treated intact plants (2 d), DW-treated infested plants (2 d) to JA-treated infested plants (2 d). Single JA-treatment to either infested or uninfested plants increased attractiveness to C. kariyai over a period lasting at least 10 d. We then showed that the increase in attractiveness of infested corn plants by JA-treatment resulted in increased parasitism by C. kariyai. These results hold a promise for field application of JA-treatment. First, JA-treatment not only promotes the attractiveness of uninfested plants, but also armyworm-infested plants. Thus, parasitoids are not likely to waste time on JA-treated uninfested plants when JA-treated infested plants are available. Second, the effect of JA-treatment is lasting for at least 10 d, a result now obtained in two independent studies.
Similar content being viewed by others
REFERENCES
Baldwin, I. T. 1998. Jasmonate-induced responses are costly but benefit plants under attack in-native populations. Proc.Natl.Acad.Sci.U.S.A. 95:8113–8118.
Benrey, B., Callejas, A., Rios, L., Oyama, K., and Denno, R. F. 1998. The effects of domestication of brassica and phaseolus on the interaction between phytophagous insects and parasitoids. Biol.Control 11:130–140.
Boland, W., Hopke, J., Donath, J., N¨uske, J., and Bublitz, F. 1995. Jasmonic acid and coronatin induce odor production in plants. Angew.Chem.Int.Ed.Engl. 34:1600–1602.
Choh, Y., Ozawa, R., and Takabayashi, J. 2004. Effects of exogenous jasmonic acid and benzo (1, 2, 3) thiadiazole-7-carbothioic acid S-methyl ester (BTH), a functional analogue of salicylic acid, on the egg production of a herbivorous mite Tetranychus urticae (Acari: Tetranychidae). Appl.Entomol.Zool. 39:313–316.
Cipollini, D. F.,Jr. and Redman, A. M. 1999. Age-dependent effects of jasmonic acid treatment and wind exposure on foliar oxidase activity and insect resistance in tomato. J.Chem.Ecol. 25:271–281.
Dicke, M. 2000. Chemical ecology of host–plant selection by herbivorous arthropods: A multitrophic perspective. Biochem.Syst.Ecol. 28:601–618.
Dicke, M., Gols, R., Ludeking, D., and Posthumus, M. A. 1999. Jasmonic acid and herbivory dif-ferentially induce carnivore-attracting plant volatiles in lima bean plants. J.Chem.Ecol. 25:1907–1922.
Gols, R., Posthumus, M. A., and Dicke, M. 1999. Jasmonic acid induces the production of gerbera volatiles that attract the biological control agent Phytoseiulus persimilis. Entomol.Exp.Appl. 93:77–86.
Gols, R., Roosjen, M., Dijkman, H., and Dicke, M. 2003. Induction of direct and indirect plant responses by jasmonic acid, lowspider mite densities, or a combination of jasmonic acid treatment and spider mite infestation. J.Chem.Ecol. 29:2651–2666.
Gonz´ alez-rodr´iguez, A., Benrey, B., Castaneda, A., and Oyama, K. 2000. Population genetic structure of Acanthoscelides abtectus and A.obvelatus (Coleoptera: Bruchidae) from wild and cultivated Phaseolus spp. (Leguminosae). Ann.Entomol.Soc.Am. 93:1100–1107.
Hopke, J., Donath, J., Blechert, S., and Boland, W. 1994. Herbivore-induced volatiles: The emission of acyclic homoterpenes from leaves of Phaseolus lunatus and Zea mays can be triggered by a â-glucosidase and jasmonic acid. FEBS Lett. 352:146–150.
Lindig, R., Espinoza, F., and Benrey, B. 1997. Phytoalexins, resistance traits, and domestication status in Phaseolus coccineus and Phaseolus lunatus. J.Chem.Ecol. 23:1997–2011.
Loughrin, J. H., Manukian, A., Heath, R., and Tumlinson, J. H. 1995. Volatiles emitted by different cotton varieties damaged by feeding beet armyworm larvae. J.Chem.Ecol. 21:1217–1227.
Ozawa, R., Arimura, G., Takabayashi, J., Shimoda, T., and Nishioka, T. 2000. Involvement of jasmonate-and salicylate-related signaling pathways for the production of specific herbivore-induced volatiles in plants. Plant Cell Physiol. 41:391–398.
Redman, A. M., Cipollini, D. F., Jr., and Schultz, E. A. 2001. Fitness costs in jasmonic acid-induced defense in tomato, Lycopersicon esculentum. Oecologia 126:380–385.
Sabelis, M. W., Janssen, A., Pallini, A., Venzon, M., Bruin, J., Drukker, B., and Scutareanu, P. 1999. Behavioural responses of predatory and herbivorous arthropods to induced plant volatiles: From evolutionary ecology to agricultural applications, pp. 269–298, in Anurag A. Agrawal, Sadik Tuzun, and Elizabeth Bent (eds. ). Induced Plant Defenses Against Pathogens and Herbivores. APS Press, The American Phytopathological Society, St. Paul, MN.
Schmelz, E. A., Alborn, H. T., Banchio, E., and Tumlinson, J. H. 2003. Quantitative relationships between induced jasmonic acid levels and volatile emission in Zea mays during Spodoptera exigua herbivory. Planta 216:665–673.
Shimoda, T., Ozawa, R., Arimura, G., Takabayashi, J., and Nishioka, T. 2002. Olfactory responses of two specialist insect predators of spider mites toward plant volatiles from lima bean leaves induced by jasmonic acid and /or methyl salicylate. Appl.Entomol.Zool. 37:535–541.
Shiojiri, K., Takabayashi, J., Yano, S., and Takafuji, A. 2000. Flight response of parasitoid toward plant–herbivore complexes: A comparative study of two parasitoid–herbivore systems on cabbage plants. Appl.Entomol.Zool. 35:87–92.
Sokal, R. R. and Rohlf, F. J. 1995. Biometry--The Principles and Practice of Statistics in Biological Research, 3rd edn. W. H. Freeman and Company, New York.
Takabayashi, J. and Dicke, M. 1996. Plant–carnivore mutualism through herbivore-induced carnivore attractants. Trends Plant Sci. 1:109–113.
Thaler, J. 1999. Jasmonate-inducible plant defences cause increased parasitism of herbivores. Nature 399:686–688.
Wasternack, C. and Parthier, B. 1997. Jasmonate-signalled plant gene expression. Trends Plant Sci. 2:302–307.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ozawa, R., Shiojiri, K., Sabelis, M.W. et al. Corn Plants Treated with Jasmonic Acid Attract More Specialist Parasitoids, Thereby Increasing Parasitization of the Common Armyworm. J Chem Ecol 30, 1797–1808 (2004). https://doi.org/10.1023/B:JOEC.0000042402.04012.c7
Issue Date:
DOI: https://doi.org/10.1023/B:JOEC.0000042402.04012.c7