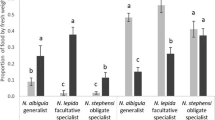
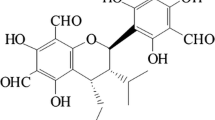
Abstract
Formylated phloroglucinol compounds (FPCs) are the single most important factor determining the amount of foliage that marsupial folivores eat from individual Eucalyptus trees. Folivores need to recognize which trees contain FPCs if they are to avoid them and forage efficiently, they are challenged by great diversity in the types and quantities of FPCs present, even within eucalypt species. We investigated the relationship between FPCs and terpenoids in species with both simple and complex FPC profiles and found strong positive correlations between terpenes generally, and several monoterpenes in particular, and FPCs. Terpene cues also indicated qualitative differences in trees' FPC profiles. We describe significant qualitative and quantitative variation in FPCs in several species that are important food sources for marsupial folivores. New discoveries include the fact that macrocarpals occur as two major, distinct groups and several new dimeric acylphloroglucinols from Eucalyptus strzeleckii. These patterns add to the chemical complexity of the foraging environment for folivores.
Similar content being viewed by others
REFERENCES
Adams, R. P. 1995. Identification of Essential Oil Components by Gas Chromatography/Mass Spec-trometry. Allured, Carol Stream, IL.
Amano, T., Komiya, T., Hori, M., Goto, M., Kozuka, M., and Sawada, T. 1981. Isolation and characterization of euglobals from Eucalyptus globulus Labill. by preparative reverse-phase liquid chromatography. J.Chromatogr. 208:347–355.
Betts, T. J. 2000. Solid phase microextraction of volatile constituents from individual fresh Eucalyptus leaves of three species. Planta Med. 66:193–195.
Bolte, M. L., Crow, W. D., Takahashi, N., Sakurai, A., Uji-ie, M., and Yoshida, S. 1985. Structure–activity relationships of grand inol--A germination inhibitor in Eucalyptus. Agric.Biol.Chem. 49:761–768.
Brophy, J. J., Goldsack, R. J., Forster, P. I., Clarkson, J. R., and Fookes, C. J. R. 1996. Mass spectra of some â-triketones from Australian Myrtaceae. J.Essent.Oil Res. 8:465–470.
Brophy, J. J. and Southwell, I. A. 2002. Eucalyptus chemistry, pp. 102–160, in J. J. W. Coppen (ed.). Eucalyptus: The Genus Eucalyptus. Taylor & Francis, London and New York.
Dewick, P. M. 2002. The biosynthesis of C5–C25 terpenoid compounds. Nat.Prod.Rep. 19:181–222.
Doran, J. C. 1992. Variation in and Breeding for Oil Yields in Leaves of Eucalyptus camaldulensis. PhD Thesis, Australian National University, Canberra.
Dungey, H. S., Potts, B. M., Whitham, T. G., and Li, H. F.2000. Plant genetics affects arthropod community richness and composition: Evidence from a synthetic eucalypt hybrid population. Evolution 54:1938–1946.
Dunlop, P. J., Bignell, C. M., and Hibbert, D. B. 2000. Use of gas chromatograms of essential leaf oils to compare clones of Eucalyptus camaldulensis. Biochem.Syst.Ecol. 28:383–391.
Eschler, B. M. and Foley, W. J. 1999. A new sideroxylonal from Eucalyptus melliodora. Aust.J.Chem. 52:157–158.
Eschler, B. M., Pass, D. M., Willis, R., and Foley, W. J. 2000. Distribution of foliar formylated phloroglucinol derivatives amongst Eucalyptus species. Biochem.Syst.Ecol. 28:813–824.
Eyles, A., Davies, N. W., and Mohammed, C. 2003. Novel detection of formylated phloroglucinol compounds (FPCs) in the wound wood of Eucalyptus globulus and E.nitens. J.Chem.Ecol. 29:881–898.
Foley, W. J., Lassak, E. V., and Brophy, J. 1987. Digestion and absorption of Eucalyptus essential oils in greater glider (Petauroides volans) and brushtail possum (Trichosurus vulpecula). J.Chem.Ecol. 13:2115–2130.
Ghisalberti, E. L. 1996. Bioactive acylphloroglucinol derivatives from Eucalyptus species. Phyto-chemistry 41:7–22.
Heller, S. R. and Milne, G. W. A. 1978, 1980, 1983. EPA/NIH Mass Spectral Database. U. S. Government Printing Office, Washington, DC.
Horn, D. H. S. and Lamberton, J. A. 1963. Nuclear magnetic resonance study of a new flavonoid. Chem.Ind. 1963:691–692.
Joulain, D. and K¨onig, W. A. 1998. The Atlas of Spectral Data of Sesquiterpene Hydrocarbons. E. B. Verlag, Hamburg, Germany.
Lawler, I. R., Eschler, B. M., Schliebs, D. M., and Foley, W. J.1999a. Relationship between chem-ical functional groups on Eucalyptus secondary metabolites and their effectiveness as marsupial antifeedants. J.Chem.Ecol. 25:2561–2573.
Lawler, I. R., Foley, W. J., and Eschler, B. M. 2000. Foliar concentration of a single toxin creates habitat patchiness for a marsupial folivore. Ecology 81:1327–1338.
Lawler, I. R., Foley, W. J., Eschler, B. M., Pass, D. M., and Hand asyde, K. 1998a. Intraspecific variation in Eucalyptus secondary metabolites determines food intake by folivorous marsupials. Oecologia 116:160–169.
Lawler, I. R., Foley, W. J., Pass, G. J., and Eschler, B. M. 1998b. Administration of a 5HT3 receptor antagonist increases the intake of diets containing Eucalyptus secondary metabolites by marsupials. J.Compar.Physiol.B, Biochem., Syst., Eupnviron.Physiol. 168:611–618.
Lawler, I. R., Stapley, J., Foley, W. J., and Eschler, B. M. 1999b. Ecological example of condi-tioned flavor aversion in plant–herbivore interactions: Effect of terpenes of Eucalyptus leaves on feeding by common ringtail and brushtail possums. J.Chem.Ecol. 25:401–415.
Legendre, P. 2001. Model II Regression--User's Guide, p. 23. D´epartement de sciences biologiques, Universit´e de Montr´eal.
Menut, C., Bessiere, J. M., Samate, A. D., Millogo-rasolodimby, J., and Nacro, M. 1999. Apodophyllone and isotorquatone, two arenic ketones from Eucalyptus apodophylla. Phyto-chemistry 51:975–978.
Mitaine-offer, A. C., D´esir´edjoukeng, J., Azefack tapondjou, L., Bouda, H., Lerche, H., Lontsi, D., and Lacaille-dubois, M. A. 2003. Constituents of the leaves of Eucalyptus saligna. Biochem.Syst.Ecol. 31:1457–1460.
Moore, B. D., Wallis, I. R., Wood, J., and Foley, W. J. in press. Foliar nutrition, site quality and temperature affect foliar chemistry of tallowwood (Eucalyptus microcorys). Ecological Monographs.
Nishizawa, M., Emura, M., Kan, Y., Yamada, H., Ogawa, K., and Hamanaka, N. 1992. Macro-carpals: HIV-reverse transcriptase inhibitors of Eucalyptus globulus. Tetrahedron Lett. 33:2983–2986.
Osawa, K., Yasuda, H., Morita, H., Takeya, K., and Itokawa, H. 1995. Eucalyptone from Euca-lyptus globulus. Phytochemistry 40:183–184.
Osawa, K., Yasuda, H., Morita, H., Takeya, K., and Itokawa, H. 1996. Macrocarpals H, I and J from the leaves of Eucalyptus globulus. J.Nat.Prod. 59:823–827.
Rule, K. 1992. Two new species of Eucalyptus (Myrtaceae) in south-eastern Australia. Muelleria 7:497–505.
Sarker, S. D., Bartholomew, B., Nash, R. J., and Simmonds, M. S. J. 2001. Sideroxylin and 8-demethylsideroxylin from Eucalyptus saligna (Myrtaceae). Biochem.Syst.Ecol. 29:759–762.
Satoh, H., Etoh, H., Watanabe, N., Kawagishi, H., Arai, K., and Ina, K. 1992. Structures of sideroxylonals from Eucalyptus sideoxylon. Chem.Lett. 1917–1920.
Shibuya, Y., Kusuoka, H., Murphy, G. K., and Nishizawa, Y. 2001. Isolation and structure de-termination of new macrocarpals from a herbal medicine, Eucalyptus globulus leaf. Nat.Med. 55:28–31.
Singh, I. P., Hayakawa, R., Etoh, H., Takasaki, M., and Konoshima, T. 1997. Grandinal, a new phloroglucinol dimer from Eucalyptus grandis. Biosci.Biotechnol.Biochem. 61:921–923.
Stenhagen, E., Abrahamsson, S., and Mclafferty, F. W. 1974. Registry of Mass Spectra Data. Wiley, New York.
Swigar, A. A. and Silverstein, R. M. 1981. Monoterpenes. Aldrich, Milwaukee, WI.
Takasaki, M., Konoshima, T., Fujitani, K., Yoshida, S., Nishimura, H., Tokuda, H., Nishino, H., Iwashima, A., and Kosuka, M. 1990. Inhibitors of skin-tumor promotion. 8: Inhibitory effects of euglobals and their related compounds on Epstein-Barr virus activation. Chem.Pharm.Bull.(Tokyo) 38:2737–2739.
Terada, Y., Saito, J., Kawai, T., Singh, I. P., and Etoh, H. 1999. Structure–activity relationship of phloroglucinol compounds from Eucalyptus, as marine antifoulants. Biosci.Biotechnol.Biochem. 63:276–280.
Wallis, I. R., Watson, M. L., and Foley, W. J. 2002. Secondary metabolites in Eucalyptus melliodora:Field distribution and laboratory feeding choices by a generalist herbivore, the common brushtail possum. Aust.J.Zool. 50:507–519.
Yamakoshi, Y., Murata, M., Shimizu, A., and Homma, S. 1992. Isolation and characterisation of Macrocarpals B-G antibacterial compounds from Eucalyptus macrocarpa. Biosci.Biotechnol.Biochem. 56:1570–1576.
Yoshida, S., Asami, T., Kawano, T., Yoneyama, K., Crow, W. D., Paton, D. M., and Takahashi, N. 1988. Photosynthetic inhibitors in Eucalyptus grandis. Phytochemistry 27:1943–1946.
Zini, C. A., Augusto, F., Christensen, E., Caramao, E. B., and Pawliszyn, J. 2002. SPME applied to the study of volatile organic compounds emitted by three species of Eucalyptus in situ. J.Agric.Food Chem. 50:7199–7205.
Zini, C. A., Zanin, K. D., Christensen, E., Caramao, E. B., and Pawliszyn, J. 2003. Solid-phase microextraction of volatile compounds from the chopped leaves of three species of Eucalyptus. J.Agric.Food Chem. 51:2679–2686.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Moore, B.D., Wallis, I.R., Palá-Paúl, J. et al. Antiherbivore Chemistry of Eucalyptus--Cues and Deterrents for Marsupial Folivores. J Chem Ecol 30, 1743–1769 (2004). https://doi.org/10.1023/B:JOEC.0000042399.06553.c6
Issue Date:
DOI: https://doi.org/10.1023/B:JOEC.0000042399.06553.c6