Abstract
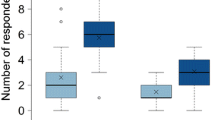
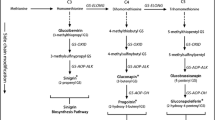
The effect of aphid population size on host-plant chemicalDefense expression and the effect of plantDefense on aphid population dynamics were investigated in a milkweed-specialist herbivore system. Density effects of the aposematic oleander aphid, Aphis nerii, on cardenolide expression were measured in two milkweed species, Asclepias curassavica and A. incarnata. These plants vary in constitutive chemical investment with high mean cardenolide concentration in A. curassavica and low to zero in A. incarnata. The second objective was toDetermine whether cardenolide expression in these two host plants impacts mean A. nerii colony biomass (mg) andDensity. Cardenolide concentration (μg/g) of A. curassavica in both aphid-treated leaves and opposite, herbivore-free leavesDecreased initially in comparison with aphid-free controls, and then increased significantly with A. neriiDensity. Thus, A. curassavica responds to aphid herbivory initially withDensity-dependent phytochemical reduction, followed by induction of cardenolides to concentrations above aphid-free controls. In addition, mean cardenolide concentration of aphid-treated leaves was significantly higher than that of opposite, herbivore-free leaves. Therefore, A. curassavica induction is strongest in herbivore-damaged tissue. Conversely, A. incarnata exhibited no such chemical response to aphid herbivory. Furthermore, neither host plant responded chemically to herbivore feeding duration time (days) or to the interaction between herbivore initialDensity and feeding duration time. There were also no significant differences in mean colony biomass or populationDensity of A. nerii reared on high cardenolide (A. curassavica) and low cardenolide (A. incarnata) hosts.
Similar content being viewed by others
References
Bailey, M. P. 1974. Cardenolides (Cardiac Glycosides) in the Food and Honeydew of the Oleander Aphid, Aphis nerii. Honors Thesis, School of Natural Sciences, University of Oxford, England.
Botha, C. E. J., Malcolm, S. B., and Evert, R. F. 1977. An investigation of preferential feeding habit in four Asclepiadaceae by the aphid, Aphis nerii B.De F. Protoplasma 92:1–19.
Bristow, C. M. 1991. Are ant-aphid associations a tritrophic interaction? Oleander aphids and Argentine ants. Oecologia 87:514–521.
Dixon, A. F. G. 1970. Quality and availability of food for a sycamore aphid population, pp. 271–286, in A. Watson (ed.). Animal Populations in Relation to Their Food Resources. Brit. Ecol. Soc. Symp. 10. Blackwell Scientific, Oxford, UK.
Dixon, A. F. G. 1998. Aphid Ecology. Chapman and Hall, London, 300p.
Duffey, S. S. 1980. Sequestration of plant natural products by insects. Annu. Rev. Entomol. 25:447–477.
Gianoli, E. and Niemeyer, H. M. 1996. Environmental effects on the induction of wheat chemicalDefences by aphid infestation. Oecologia 107:549–552.
Gianoli, E. and Niemeyer, H. M. 1997. Characteristics of hydroxamic acid induction in wheat triggered by aphid infestation. J. Chem. Ecol. 23:2695–2705.
Groeters, F. R. 1989. Geographical and colonial variation in the milkweed-oleander aphid A. nerii for winged morph production, life history and morphology in relation to host plant permanence. Evol. Ecol. 3:327–341.
Groeters, F. R. and Dingle, H. A. 1989. The cost of being able to fly in the milkweed-oleander aphid, Aphis nerii (Homoptera: Aphididae). Evol. Ecol. 3(3):313–326.
Hall, R. W. and Ehler, L. E. 1980. Population ecology of Aphis nerii on oleander. Environ. Entomol. 9(3):338–344.
Hunter, M. D. and Price, P. W. 1992. Playing chutes and ladders: Heterogeneity and the relative roles of bottom-up and top-down forces in natural communities. Ecology 73:724–732.
Ismail, I. I. and Swailem, S. M. 1971. Seasonal occurrence of Aphis nerii Boyer in the Giza region (Egypt) (Hemiptera - Homoptera: Aphididae). Bull. Soc. ent. Égypte 55:231–238.
Karban, R. and Baldwin, I. T. 1997. Induced Responses to Herbivory. Chicago University Press, Chicago.
Malcolm, S. B. 1976. An Investigation of Plant-Derived Cardiac Glycosides as a Possible Basis for Aposematism in the Aphidophagous Hoverfly Ischiodon aegyptius (Wiedemann) (Diptera: Syrphidae). MSc Dissertation, Rhodes University, Republic of South Africa.
Malcolm, S. B. 1981. Defensive Use of Plant-Derived Cardenolides by Aphis nerii BoyerDe Fonscolombe Against Predation. DPhil Dissertation, University of Oxford, England.
Malcolm, S. B. 1986. Aposematism in a soft-bodied insect: A case for kin selection. Behav. Ecol. Sociobiol. 18:387–393.
Malcolm, S. B. 1989. Disruption of web structure and predatory behavior of a spider by plant-derived chemicalDefenses of an aposematic aphid. J. Chem. Ecol. 15:1699–1716.
Malcolm, S. B. 1990. ChemicalDefence in chewing and sucking insect herbivores: Plant-derived cardenolides in the monarch butterfly and oleander aphid. Chemoecology 1:12–21.
Malcolm, S. B. 1991. Cardenolide-mediated interactions between plants and herbivores, pp. 251–296, in G. A. Rosenthal and M. R. Berenbaum (eds.). Herbivores: Their Interactions with Secondary Plant Metabolites. Vol. I: The Chemical Participants, 2nd edn. Blackwell Scientific, Oxford, UK.
Malcolm, S. B. 1992. PreyDefence and predator foraging, pp. 458–475, in M. J. Crawley (ed.). Natural Enemies. The Population Biology of Predators, Parasites, and Diseases. Blackwell Scientific, Oxford, UK.
Malcolm, S. B. 1995. Milkweeds, monarch butterflies and the ecological significance of cardenolides. Chemoecology 5/6:101–117.
Malcolm, S. B. and Zalucki, M. P. 1996. Milkweed latex and cardenolide induction may resolve the lethal plantDefence paradox. Entomol. Exp. Appl. 80:193–196.
McNeill, S. and Southwood, T. R. E. 1978. The role of nitrogen in theDevelopment of insect/plant relationships, pp. 77–98, in J. B. Harborne (ed.). Biochemical Aspects of Plant and Animal Coevolution. Academic Press, London.
Muller, K. O. 1959. Hypersensitivity, pp. 469–519, in J.G. Horsfall and A. E. Dimond (eds.). Plant Pathology: An Advanced Treatise. Academic Press, New York.
Pasteels, J. M. 1978. Apterous and brachypterous coccinellids at the end of the food chain, Cionura erecta (Asclepiadaceae) - Aphis nerii. Entomol. Exp. Appl. 24:379–384.
Patch, E. M. 1938. Food-plant catalogue of the aphids of the world. Including the Phylloxeridae. Bull. Maine Agr. Exp. Stn. 393:341.
Price, P. W. and Willson, M. F. 1979. Abundance of herbivores on six milkweed species in Illinois. American Midland Naturalist 101:76–86.
Roeske, C. N., Sieber, J. N., Brower, L. P., and Moffitt, C. M. 1976. Milkweed cardenolides and their comparative processing by monarch butterflies (Danaus plexippus L.). Rec. Adv. Phytochem. 10:93–167.
Rothschild, M., von Euw, J., and Reichstein, T. 1970. Cardiac glycosides in the oleander aphid Aphis nerii. J. Insect Physiol. 16:1191–1195.
Woodson, R. E. Jr. 1954. The North American species of Asclepias L. Ann. Mo. Bot. Gard. 41:1–211.
Wiegrebe, H. and Wichtl, M. 1993. High-performance liquid chromatographicDetermination of cardenolides in Digitalis leaves after solid-phase extraction. J. Chromatogr. 630:402–407.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Martel, J.W., Malcolm, S.B. Density-Dependent Reduction and Induction of Milkweed Cardenolides by a Sucking Insect Herbivore. J Chem Ecol 30, 545–561 (2004). https://doi.org/10.1023/B:JOEC.0000018628.48604.79
Issue Date:
DOI: https://doi.org/10.1023/B:JOEC.0000018628.48604.79