Abstract
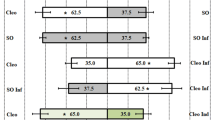
Many carnivorous arthropods use herbivore-induced plant volatiles to locate their prey. These plant volatiles are blends of up to hundreds of compounds. It is often unknown which compounds in such a complex volatile blend represent the signal to the foraging carnivore. We studied the role of methyl salicylate (MeSA) as part of the volatile blend in the foraging behavior of the predatory mite Phytoseiulus persimilis by using a Y-tube olfactometer. MeSA is one of the compounds released by lima bean, infested with Tetranychus urticae—a prey species of the predatory mite. MeSA attracted satiated predatory mites in a dose-dependent way with optimum attraction at a dose of 0.2 μg. Predatory mites did not discriminate between a prey-induced lima bean volatile blend (that contains MeSA) and a prey-induced volatile blend to which an extra amount of synthetic MeSA had been added. However, they preferred a MeSA-containing volatile blend (induced by T. urticae) to an otherwise similar but MeSA-free blend (induced by jasmonic acid). Adding synthetic MeSA to the MeSA-free blend significantly increased the mites' choice for this odor, suggesting an important role for MeSA. This study is a new step toward unraveling the role of herbivore-induced plant volatiles in the foraging behavior of predatory arthropods.
Similar content being viewed by others
REFERENCES
Bernays, E. A. 2001. Neural limitations in phytophagous insects: Implications for diet breadth and evolution of host affiliation. Annu. Rev. Entomol. 46:703–727.
Bernays, E. A. and Wcislo, W. T. 1994. Sensory capabilities, information processing, and resource specialization. Q. Rev. Biol. 69:187–204.
Cardoza, Y. J., Alborn, H. T., and Tumlinson, J. H. 2002. In vivo volatile emissions from peanut plants induced by simultaneous fungal infection and insect damage. J. Chem. Ecol. 28:161–174.
Crawley, M. J. 1993. GLIM for ecologists, pp. 265–290, in J. H. Lawton and G. E. Likens (eds.). Methods in Ecology. Blackwell, Oxford.
De Bruyne, M., Dicke, M., and Tjallingii, W. F. 1991. Receptor cell responses in the anterior tarsi of Phytoseiulus persimilis to volatile kairomone components. Exp. Appl. Acarol. 13:53–58.
De Moraes, C. M., Lewis, W. J., Paré, P. W., Alborn, H. T., and Tumlinson, J. H. 1998. Herbivore-infested plants selectively attract parasitoids. Nature 393:570–573.
Dicke, M. 1999. Specificity of herbivore-induced plant defences, pp. 43–54, in D. J. Chadwick and J. Goode (eds.). Insect-Plant Interactions and Induced Plant Defence (Novartis Foundation Symposium 223). Wiley, Chicester, UK.
Dicke, M. and Van Loon, J. J. A. 2000. Multitrophic effects of herbivore-induced plant volatiles in an evolutionary context. Entomol. Exp. Appl. 97:237–249.
Dicke, M. and Vet, L. E. M. 1999. Plant-carnivore interactions: Evolutionary and ecological consequences for plant, herbivore and carnivore, pp. 483–520, in H. Olff, V. K. Brown, and R. H. Drent (eds.). Herbivores: Between Plants and Predators. Blackwell, Oxford.
Dicke, M., Gols, R., Ludeking, D., and Posthumus, M. A. 1999. Jasmonic acid and herbivory differentially induce carnivore-attracting plant volatiles in Lima bean plants. J. Chem. Ecol. 25:1907–1922.
Dicke, M., Takabayashi, J., Posthumus, M. A., Schutte, C., and Krips, O. E. 1998. Plant-phytoseiid interactions mediated by herbivore-induced plant volatiles: Variation in production of cues and in responses of predatory mites. Exp. Appl. Acarol. 22:311–333.
Dicke, M., Van Beek, T. A., Posthumus, M. A., Ben Dom, N., Van Bokhoven, H., and De Groot, A. E. 1990. Isolation and identification of volatile kairomone that affects acarine predator-prey interactions. Involvement of host plant in its production. J. Chem. Ecol. 16:381–396.
Drukker, B., Bruin, J., Jacobs, G., Kroon, A., and Sabelis, M. W. 2000. How predatory arthropods learn to cope with variability in volatile plant signals in the environment of their herbivore prey. Exp. Appl. Acarol. 24:881–895.
Du, Y. J., Poppy, G. M., Powell, W., Pickett, J. A., Wadhams, L. J., and Woodcock, C. M. 1998. Identification of semiochemicals released during aphid feeding that attract parasitoid Aphidius ervi. J. Chem. Ecol. 24:1355–1368.
Geervliet, J. B. F., Posthumus, M. A., Vet, L. E. M., and Dicke, M. 1997. Comparative analysis of headspace volatiles from different caterpillar-infested or uninfested food plants of Pieris species. J. Chem. Ecol. 23:2935–2954.
Gouinguené, S. P. and Turlings, T. C. J. 2002. The effects of abiotic factors on induced volatile emissions in corn plants. Plant Physiol. 129:1296–1307.
Gouinguené, S., Degen, T., and Turlings, T. C. J. 2001. Variability in herbivore-induced odour emissions among maize cultivars and their wild ancestors (teosinte). Chemoecology 11:9–16.
Helle, W. and Sabelis, M. W. (eds). 1985. World Crop Pests: Spider Mites. Their Biology, Natural Enemies and Control. Vol. 1B. Elsevier, Amsterdam, The Netherlands.
Krips, O. E., Willems, P. E. L., Gols, R., Posthumus, M. A., and Dicke, M. 1999. The response of Phytoseiulus persimilis to spider mite-induced volatiles from gerbera: Influence of starvation and experience. J. Chem. Ecol. 25:2623–2641.
Li, Y., Dickens, J. C., and Steiner, W. W. M. 1991. Antennal olfactory responsiveness of Microplitis croceipes (Hymenopetera: Braconidae) to cotton plant volatiles. J. Chem. Ecol. 18:1761–1773.
Ozawa, R., Arimura, G., Takabayashi, J., Shimoda, S., and Nishioka, T. 2000. Involvement of jasmonate-and salicylate-related signaling pathways for the production of herbivore-induced volatiles in plants. Plant Cell Physiol. 41:391–398.
Paré, P. W. and Tumlinson, J. H. 1999. Plant volatiles as a defence against insect herbivores. Plant Physiol. 121:325–331.
Pickett, J. A., Wadhams, L. J., and Woodcock, C. M. 1998. Insect supersense. Mate and host location by insects as model systems for exploiting olfactory interactions. The Biochemist 8–13.
Sabelis, M. W. and Van De Baan, H. E. 1983. Location of distant spider mite colonies by phytoseiid predators: Demonstration of specific kairomones emitted by Tetranychus urticae and Panonychus ulmi. Entomol. Exp. Appl. 33:303–314.
Sabelis, M. W., Vermaat, J. E., and Groeneveld, A. 1984. Arrestment responses of the predatory mite, Phytoseiulus persimilis, to steep odour gradients of a kairomone. Physiol. Entomol. 9:437–446.
Scutareanu, P., Drukker, B., Bruin, J., Posthumus, M. A., and Sabelis, M. W. 1997. Volatiles from Psylla-infested pear trees and their possible involvement in attraction of anthocorid predators. J. Chem. Ecol. 23:2241–2260.
Shimoda, T. and Dicke, M. 2000. Attraction of a predator to chemical information related to nonprey: when can it be adaptive? Behav. Ecol. 11:606–613.
Smid, H. M., Van Loon, J. J. A., Posthumus, M. A., and Vet, L. E. M. 2002. GC-EAG-analysis of volatiles from Brussels sprouts plants damaged by two species of Pieris caterpillars: Olfactory receptive range of a specialist and a generalist parasitoid wasp species. Chemoecology 12:169–176.
Takabayashi, J. and Dicke, M. 1992. Response of predatory mites with different rearing histories to volatiles of uninfested plants. Entomol. Exp. Appl. 64:187–193.
Takabayashi, J., Dicke, M., and Posthumus, M. A. 1994a. Volatile herbivore-induced terpenoids in plant-mite interactions: Variation caused by biotic and abiotic factors. J. Chem. Ecol. 20:1329–1354.
Takabayashi, J., Dicke, M., Takahashi, S., Posthumus, M. A., and Van Beek, T. A. 1994b. Leaf age affects composition of herbivore-induced synomones and attractions of predatory mites. J. Chem. Ecol. 20:373–386.
Takabayashi, J., Takahashi, S., Dicke, M., and Posthumus, M. A. 1995. Developmental stage of herbivore Pseudaletia separata affects production of herbivore-induced synomone by corn plants. J. Chem. Ecol. 21:273–287.
Turlings, T. C. J. and Fritzsche, M. E. 1999. Attraction of parasitic wasps by caterpillar-damaged plants, pp. 21–32, in D. J. Chadwick and J. Goode (eds.). Insect-Plant Interactions and Induced Plant Defence (Novartis Foundation Symposium 223). Wiley, Chicester, UK.
Turlings, T. C. J., Bernasconi, M., Bertossa, R., Bigler, F., Caloz, G., and Dorn, S. 1998. The induction of volatile emissions in maize by three herbivore species with different feeding habits: Possible consequences for their natural enemies. Biol. Contr. 11:122–129.
Turlings, T. C. J., McCall, P., Alborn, H. T., and Tumlinson, J. H. 1993. An elicitor in caterpillar oral secretions that induces corn seedlings to emit chemical signals attractive to parasitic wasps. J. Chem. Ecol. 19:411–425.
Turlings, T. C. J., Tumlinson, J. H., Heath, R. R., Proveaux, A. T., and Doolittle, R. E. 1991. Isolation and identification of allelochemicals that attract the larval parasitoid, Cotesia marginiventris (Cresson), to the microhabitat of its hosts. J. Chem. Ecol. 17:2235–2251.
Van den Boom, C. E. M., Van Beek, T. A., Posthumus, M. A., De Groot, A. E., and Dicke, M. in press. Qualitative and quantitative variation between volatile profiles induced by Tetranychus urticae feeding on different plant parts of various families. J. Chem. Ecol. 30:69–90.
Vet, L. E. M. 1999a. From chemical to population ecology: Infochemical use in an evolutionary context. J. Chem. Ecol. 25:31–49.
Vet, L. E. M. 1999b. Evolutionary aspects of plant-carnivore interactions. pp. 3–20, in D. J. Chadwick and J. A. Goode (eds.). Insect-Plant Interactions and Induced Plant Defence (Novartis Foundation Symposium 223). Wiley, Chicester, UK.
Vet, L. E. M. and Dicke, M. 1992. Ecology of infochemical use by natural enemies in a tritrophic context. Annu. Rev. Entomol. 37:141–172.
Vet, L. E. M., De J, A. G., Franchi, E., and Papaj, D. R. 1998. The effect of complete versus incomplete information on odour discrimination in a parasitic wasp. Anim. Behav. 55:1271–1279.
Weissbecker, B., Van Loon, J. J. A., Posthumus, M. A., Bouwmeester, H. J., and Dicke, M. 2000. Identification of volatile potato sesquiterpenoids and their olfactory detection by the two-spotted stinkbug Perillus bioculatus. J. Chem. Ecol. 26:1433–1445.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
De Boer, J.G., Dicke, M. The Role of Methyl Salicylate in Prey Searching Behavior of the Predatory Mite Phytoseiulus persimilis . J Chem Ecol 30, 255–271 (2004). https://doi.org/10.1023/B:JOEC.0000017976.60630.8c
Issue Date:
DOI: https://doi.org/10.1023/B:JOEC.0000017976.60630.8c