Abstract
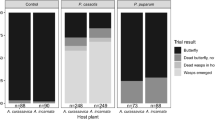
Recent studies demonstrate that generalist insect herbivores may be more subject to predation than specialists and suggest that this pattern is related to the availability and utilization of plant-derived natural products in diets of the latter. This intuitively attractive hypothesis has seldom been tested under natural conditions, however. A well-described plant-insect system, the buckeye butterfly, Junonia coenia, and its iridoid glycoside-containing host plants, was used to test the hypotheses: (1) that larval sequestration of iridoids reduces predation in the field, and (2) that this protection varies among populations. Fifth-instars reared in the laboratory on leaves of two host-plant species, Kickxia elatine (Scrophulariaceae) or Plantago lanceolata (Plantaginaceae), or on artificial diets were attached to monofilament tethers and set out into four natural populations. Only larvae fed on the locally available host plant and artificial diet-fed caterpillars were used at each site. Despite high overall predation, significantly more leaf-fed caterpillars survived than did artificial diet-fed caterpillars in Plantago sites, but the trend was not significant in Kickxia sites. The results reflect the differences in iridoid glycoside availability in these two host-plant species. They indicate that predators exert a strong selective force on this insect, that sequestered iridoid glycosides can be effective deterrents to predation, and that host-plant choices by ovipositing females and feeding larvae have consequences for mortality from natural enemies. This protection, however, is not absolute, and depends on local predator abundance and/or selectivity. The ecological interactions between buckeye caterpillars, their host plants, and their natural enemies are locally variable in nature.
Similar content being viewed by others
REFERENCES
ADLER, L. S., SCHMITT, J. A., and BOWERS, M. D. 1994. Genetic variation in defensive chemistry in Plantago lanceolata (Plantaginaceae) and its effect on the specialist herbivore Junonia coenia (Nymphalidae). Oecologia 101:75–85
BELOFSKY, G., BOWERS, M. D., JANZEN, S., and STERMITZ, F. R. 1989. Iridoid glycosides of Aureolaria flava and their sequestration by Euphydryas phaeton butterflies. Phytochemistry 28:1601–1604
BERNAYS, E., and GRAHAM, M. 1988. On the evolution of host specificity in phytophagous arthropods. Ecology 69:886–892
BERNAYS, E. A. 1988. Host specificity in phytophagous insects: Selection pressure from generalist predators. Entomol. Exp. Appl. 49:131–140
BERNAYS, E. A., and CORNELIUS, M. L. 1989. Generalist caterpillar prey are more palatable than specialists for the generalist predator Iridomyrmex humilis. Oecologia 79:427–430
BLUM, M. S. 1981. The Chemical Defenses of Arthropods. Academic Press, New York
BOWERS, M. D. 1980. Unpalability as a defensive strategy of Euphydryas phaeton (Lepidoptera: Nymphalidae). Evolution 34:586–600
BOWERS, M. D. 1981. Unpalatability as a defense strategy of western checkerspot butterflies (Euphydryas). Evolution 35:367–375
BOWERS, M. D. 1984. Iridoid glycosides and host-plant specificity in larvae of the buckeye butterfly, Junonia coenia (Nymphalidae). J. Chem. Ecol. 10:1567–1577
BOWERS, M. D. 1990. Recycling plant natural products for insect defense, pp. 353–386, in D. L. Evans and J. O. Schmidt (eds.). Insect Defenses: Adaptive Mechanisms and Strategies of Prey And Predators SUNY Press, Stony Brook, New York
BOWERS, M. D. 1991. Iridoid glycosides, pp. 297–325, in G. A. Rosenthal and M. R. Berenbaum (eds.). Herbivores: Their Interactions with Secondary Plant Metabolites, Vol I, The Chemical Participants, 2nd ed. Academic Press, San Diego
BOWERS, M. D. 1993. Aposematic caterpillars: life-styles of the warningly colored and unpalatable, pp. 331–371, in N. E. Stamp and T. M. Casey (eds.). Caterpillars: Ecological and Evolutionary Constraints on Foraging. Chapman and Hall, New York
BOWERS, M. D., and COLLINGE, S. K. 1992. Fate of iridoid glycosides in different life stages of the buckeye, Junonia coenia, (Lepidoptera: Nymphalidae). J. Chem. Ecol. 18:817–831
BOWERS, M. D., and FARLEY, S. 1990. The behavior of grey jays, Perisoreus canadensis, towards palatable and unpalatable Lepidoptera. Anim. Behav. 39:699–705
BOWERS, M. D., and LARIN, Z. 1989. Acquired chemical defense in the lycaenid butterfly, Eumaeus atala. J. Chem. Ecol. 15:1133–1146
BOWERS, M. D., and PUTTICK, G. M. 1986. The fate of ingested iridoid glycosides in lepidopteran herbivores. J. Chem. Ecol. 12:169–178
BOWERS, M. D., STAMP, N. E., and COLLINGE, S. K. 1992. Early stage of host range expansion in a specialist herbivore. Ecology 73:526–536
BROWER, J. V. Z. 1958a. Experimental studies of mimicry in some North American butterflies. Part 1. The monarch Danaus plexippus, and the viceroy, Limenitis archippus archippus. Evolution 12:32–47
BROWER, J. V. Z. 1958b. Experimental studies of mimicry in some North American butterflies. Part II. Battus philenor and Papilio troilus, P. polyxenes, and P. glaucus. Evolution 12:123–136
BROWER, J. V. Z. 1958c. Experimental studies of mimicry in some North American butterflies. Part III. Danaus gilippus berenice and Limenitis archippus floridensis. Evolution 12:273–285
BROWER, L. P., and BROWER, J. V. Z. 1964. Birds, butterflies and plant poisons: A study in ecological chemistry. Zoologica 49:137–159
CAMARA, M. D. 1997. A recent host range expansion in Junonia coenia Hübner (Nymphalidae): Oviposition preference, survival, growth, and chemical defense. Evolution 51:873–884
COPPINGER, R. P. 1969. The effect of experience and novelty on avian feeding behaviour with reference to the evolution of warning coloration in butterflies. I. Reactions of wild caught adult blue jays to novel insects. Behaviour 35:45–60
COPPINGER, R. P. 1970. The effects of experience and novelty on avian feeding behavior with reference to the evolution of warning coloration in butterflies. II. Reactions of naive birds to novel insects. Am. Nat. 104:323–334
DE LA FUENTE, M., DYER, L. A., and BOWERS, M. D. 1994/1995. The iridoid glycoside, eatalpol, as a deterrent to the predator Camponotus floridanus (Formicidae). Chemoecology 5/6:13–18
DEMPSTER, J. P. 1983. The natural control of populations of butterflies and moths. Biol. Rev. 58:461–481
DENNO, R. F., LARSSON, S., and OLMSTEAD, K. L. 1990. Role of enemy-free space and plant quality in host-plant selection by willow beetles. Ecology 71:124–137
DUFFEY, S. S. 1980. Sequestration of plant natural products by insects. Annu. Rev. Entomol. 25:447–477
DUNCAN, C. J., and SHEPPARD, P. M. 1965. Sensory discrimination and its role in the evolution of batesian mimicry. Behaviour 24:269–282
DYER, L. A. 1994. Lepidopteran larval defenses against predators in tropical and temperate systems: The importance of diet breadth and chemistry. PhD dissertation. University of Colorado, Boulder
DYER, L. A., and FLOYD, T. 1993. Determinants of predation in phytophagous insects: The importance of diet breadth. Oecologia 96:575–582
GARDNER, D. R. and STERMITZ, F. R. 1988. Hostplant utilization and iridoid glycoside sequestration by Euphydryas anicia individuals and populations. J. Chem. Ecol. 14:2147–2168
GITTLEMAN, J. L. P., HARVEY, P. H., and GREENWOOD, P. J. 1980. The evolution of conspicuous coloration: some experiments in bad taste. Anim. Behav. 28:897–899
JARVI, T., SILLEN TULLBERG, B., and WIKLUND, C. 1981. The cost of being aposematic. An experimental study of predation on larvae of Papilio machon by the great tit, Parus major. Oikos 36:267–272
KEIPER, R. 1969. Causal factors of stereotypies in caged birds. Anim. Behav. 17:114–119
LEA, R. G., and TURNER, J. R. G. 1972. Experiments on mimicry: II. The effect of a Batesian mimic on its model. Behaviour 42:131–151
MONTLLOR, C. B., and BERNAYS, E. A. 1993. Invertebrate predators and caterpillar foraging. pp. 170–202, in N. E. Stamp and T. M. Casey (eds.). Caterpillars: Ecological and Evolutionary Constraints on Foraging. Chapman and Hall, New York
MORRELL, G. M., and TURNER, J. R. G. 1970. Experiments on mimicry: I. The response of wild birds to artificial prey. Behaviour 36:116–131
MORRIS, R. F. 1972. Predation by wasps, birds, and mammals on Hyphantria cunea. Can. Entomol. 104:1581–1591
PEREYRA, P. C., and BOWERS, M. D. 1988. Iridoid glycosides as oviposition stimulants for the buckeye butterfly, Junonia coenia (Nymphalidae). J. Chem. Ecol. 14:917–928
SARGENT, T. D. 1967. Stereotypies in caged birds. Anim. Behav. 15:62–66
SAS Institute. 1987. SAS/STAT Guide for Personal Computers, Version 6 Edition. SAS Institute Inc., Cary, North Carolina.
STAMP, N. E. 1992. Susceptibility of specialist versus generalist caterpillars to invertebrate predators. Oecologia 92:124–129
STAMP, N. E., and BOWERS, M. D. 1992. Behaviour of specialist and generalist caterpillars on plantain (plantago lanceolata). Ecol. Entomol. 17:273–279
STEWARD, V. B., SMITH, K. G., and STEPHEN, F. M. 1988. Predation by wasps on lepidopteran larvae in an Ozark forest canopy. Ecol. Entomol. 13:81–86
THOMPSON, J. N. 1994. The Coevolutionary Process. University of Chicago Press, Chicago
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Camara, M.D. Predator Responses to Sequestered Plant Toxins in Buckeye Caterpillars: Are Tritrophic Interactions Locally Variable?. J Chem Ecol 23, 2093–2106 (1997). https://doi.org/10.1023/B:JOEC.0000006431.34359.c2
Issue Date:
DOI: https://doi.org/10.1023/B:JOEC.0000006431.34359.c2