Abstract
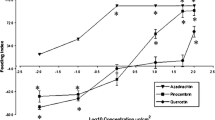
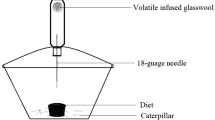
Behavioral evaluation of the antifeedant effect of 10 naturally occurring 1,4-naphthoquinones on larvae of the Mexican bean beetle, Epilachna varivestis Mulsant, was undertaken concurrently with that of a series of synthetic analogs and model compounds in order to assess structure–activity relationships. Plumbagin, 1,4-naphthoquinone, juglone, menadione, and naphthazarin, which were found to be active at 0.3% concentrations, were also bioassayed at 0.1, 0.05, and 0.01% at which concentration 1,4-naphthoquinone still retained some activity. The model studies suggest that two structural features might be operative independently against E. varivestis: one consisting of a properly substituted naphthoquinone moiety and the other requiring a benzo- or naphthohydroquinone. Within the naphthoquinone group, the relative activity is determined by a substituent effect which is the outcome of a complex interplay of electronic, steric, electrochemical, and positional requirements. Among the model compounds, 2-chloro-3-amino-1,4-naphthoquinone and α-naphthylamine displayed appreciable activity even at 0.01% The results should enable selection of plant sources for naphthoquinones possessing larval inhibition properties.
Similar content being viewed by others
REFERENCES
AMBROGI, V., ARTINI, D., DE CARNERI, I., CASTELLINO, S., DRADI, E., LOGEMANN, W., MEINARDI, G., DI SOMMA, M., TOSOLINI, G., and VECCHI, E. 1970. Studies on the antibacterial and antifungal properties of 1,4-naphthoquinones. Br. J. Pharmac. 40:871–880.
ASCHER, K. R. S., and NISSIM, S. 1965. Quantitative aspects of antifeeding: Comparing “antifeedants” by assay with P. litura. Int. Pest Control 7:21–23.
ASCHER, K. R. S., ELIYAHU, M., GLOTTER, E., GOLDMAN, A., KIRSON, I., ABRAHAM, A., JACOBSON, M., and SCHMUTTERER, H. 1987. The antifeedant effect of some new withanolides on three insect species, Spodoptera littoralis, Epilachna varivestis and Tribolium castaneum. Phytoparasitica 15:15–29.
CLARK, N. G. 1985. The fungicidal activity of substituted 1,4-naphthoquinones. III. Amino, anilino and acylamino derivatives. Pestic. Sci. 16:23–32.
CURRIE, D. J., and HOLMES, H. L. 1966. Polarographic half-wave potentials of some 1,4-naphthoquinones. Can. J. Chem. 44:1027–1029.
DESMARCHELIER, J. M., and FUKUTO, T. R. 1974. Toxicological effects produced by some 1,3-benzodioxoles, catechols, and quinones in Culex mosquito larvae. J. Econ. Entomol. 67:153–158.
DETTNER, K. 1993. Dabbing and shooting of benzo-and naphthoquinone secretions: defensive strategies of bark-inhabiting aleocharine (Col.: Staphylinidae) and tenebrionid (Col.: Tenebrionidae) beetle larvae. J. Chem. Ecol. 19:1337–1354.
FIELDGATE, D. M., and WOODCOCK, D. 1973. Fungicidal activity and chemical constitution. XX. The activity of substituted 1,4-naphthohydroquinone esters and substituted 1,4-naphthoquinones against powdery mildews. Pestic. Sci. 4:193–200.
FIESER, L. F. 1948. Naphthoquinones antimalarials. III. Diene synthesis of 1,4-naphthoquinones. J. Am. Chem. Soc. 70:3165–3174.
FIESER, L. F., and FIESER, M. 1935. The reduction potentials of various naphthoquinones. J. Am. Chem. Soc. 57:491–494.
GUJAR, G. T., and MEHROTRA, K. N. 1988. Toxicity and morphogenetic effects of plumbagin on Dysdercus koenigii F. (Het., Pyrrhocoridae). J. Appl. Entomol. 105:466–470.
HASSANALI, A., and LWANDE, W. 1989. Antipest secondary metabolites from African plants, pp. 78–94, in J. T. Arnason, B. J. R. Philogène, and P. Morand (eds.). Insecticides of Plant Origin, ACS Symposium Series 387. Washington, DC, American Chemical Society.
HEDIN, P. A., COLLUM, D. H., LANGHANS, V. E., and GRAVES, C. H. 1980. Distribution of juglone and related compounds in pecan and their effect on Fusicladium effusum. J. Agr. Food Chem. 28:340–342.
HOLMES, H. L., CURRIE, D. J., MALTMAN, J. R., SILVER, R. F., LOUGH, C. E., and LESKY, G. J. 1964. Evidence for the mode of chemical action of 1,4-naphthoquinones in bacteriostasis. Chemiotherapia 9:241–247.
HOWLAND, J. L. 1963. Uncoupling and inhibition of oxidative phosphorylation by 2-hydroxy-3-alkyl-1,4-naphthoquinones. Biochim. Biophys. Acta 77:659–662.
JACOBSEN, N., and PEDERSEN, L.-E. K. 1986. Activity of 2-(1-alkenyl)-3-hydroxy-1,4-naphthoquinones and related compounds against Musca domestica. Pestic. Sci. 17:511–516.
JOSHI, N. K., and SEHNAL, F. 1989. Inhibition of ecdysteroid production by plumbagin in Dysdercus cingulatus. J. Insect Physiol. 35:737–741.
KOGAN, M. 1977. Epilachna varivestis Muls., pp. 391–394, in J. Kranz, H. Schmutterer, and W. Koch (eds.). Diseases, Pests and Weeds in Tropical Crops. Paul Parey, Berlin.
KUBO, I., UCHIDA, M., and KLOCKE, J. A. 1983. An insect ecdysis inhibitor from the African medicinal plant Plumbago capensis (Plumbaginaceae): A naturally occurring chitin synthetase inhibitor. Agr. Biol. Chem. 47:911–913.
MARMOR, S. 1963. The epoxidation of certain α,β-unsaturated ketones with sodium hypochlorite. J. Org. Chem. 28:250–251.
MARSTON, A., MSONTHI, J. D., and HOSTETTMANN, K. 1984. Naphthoquinones of Diospyros usambarensis; Their molluscicidal and fungicidal activities. Planta Med. 50:279–280.
MITCHELL, M. J., and SMITH, S. L. 1988. Effects of the chitin synthetase inhibitor plumbagin and its 2-demethyl derivative juglone on insect ecdysone 20-monooxygenase activity. Experientia 44:990–991.
MOORE, R. E., and SCHEUER, P. J. 1966. Nuclear magnetic resonance spectra of substituted naphthoquinones. Influence of substitutents on tautomerism, anisotropy, and stereochemistry in the naphthazarin system. J. Org. Chem. 31:3272–3283.
MOORE, R. E., SINGH, H., CHANG, C. W. J., and SCHEUER, P. J. 1966. Sodium borohydride reduction of Spinochrome A. Removal of phenolic hydroxyls in the naphthazarin system. J. Org. Chem. 31:3638–3645.
MÜLLER, W.-U., and LEISTNER, E. 1976. 1,4-Naphthoquinone, an intermediate in juglone (5-hydroxy-1,4-naphthoquinone) biosynthesis. Phytochemistry 15:407–410.
NORRIS, D. M. 1969. Transduction mechanism in olfaction and gustation. Nature 222:1263–1264.
NORRIS, D. M. 1986. Anti-feeding compounds, pp. 97–146, in W. S. Bowers, W. Ebing, T. R. Fukuto, D. Martin, R. Wegler, and I. Yamamoto (eds.). Chemistry of Plant Protection 1. Sterol Biosynthesis, Inhibitors and Anti-Feeding Compounds, Springer-Verlag, Berlin/Heidelberg.
NORRIS, D. M. 1988. Periplaneta americana perception of phytochemical naphthoquinones as allelochemicals. J. Chem. Ecol. 14:1807–1819.
NORRIS, D. M., FERKOVICH, S. M., ROZENTAL, J. M., BAKER, J. E., and BORG, T. K. 1970. Energy transduction: inhibition of cockroach feeding by naphthoquinones. Science 170:754–755.
PAPAGEORGIOU, V. P. 1980. Carbon-13 NMR spectra of some naturally occurring hydroxynaphtha-quinones. Planta Med. 40:305–307.
REED, D. K., JACOBSON, M., WARTHEN, J. D., JR., UEBEL, E. C., TROMLEY, N. J., JURD, L., and FREEDMAN, B. 1981. Cucumber beetle antifeedants: Laboratory screening of natural products. Tech. Bull. No. 1641, USDA-SEA, Beltsville, MD.
RODDICK, J. G., RIJNENBERG, A. L., and OSMAN, S. F. 1988. Synergistic interaction between potato glycoalkaloids α-solanine and α-chaconine in relation to destabilization of cell membranes: Ecological implications. J. Chem. Ecol. 14:889–902.
SANKAR, R., DEVAMANOHARAN, P. S., RAGUPATHI, G., and SHYAMALA DEVI, C. S. 1987. Antilipid peroxidative efficacy of plumbagin and menadione: An in vitro study. Curr. Sci. 56:890–892.
SANKARAM, A. V. B., SRINIVASAN, T. N., and INDULKAR, A. S. 1975. Fungicidal activity of some naturally occurring naphthoquinones. Pestic. Sci. 6:165–168.
SAXENA, B. P., and TIKKU, K., 1988. Exploitation of lacunae by some allelochemics in insect-plant interactions, pp. 105–122, in T. N. Ananthakrishnan and A. Raman (eds.). Dynamics of Insect-Plant Interaction. Oxford IBH, New Delhi, India.
SINGH, I., OGATA, R. T., MOORE, R. E., CHANG, C. W. J., and SCHEUER, P. J. 1968. Electronic spectra of substituted naphthoquinones. Tetrahedron 24:6053–6073.
SPENCER, G. F., TJARKS L. W., ENGLAND, R. E., and SEEST, E. P. 1986. The effect of naturally occurring naphthoquinones on velvetleaf (Abutilon theophrasti) germination. J. Nat. Prod. 49:530–533.
THIBOLDEAUX, R. L., LINDROTH, R. L., and TRACY, J. W. 1994. Differential toxicity of juglone (5-hydroxy-1,4-naphthoquinone) and related naphthoquinones to saturniid moths. J. Chem. Ecol. 20:1631–1641.
THOMSON, R. H. 1971. Naturally Occurring Quinones, 2nd ed. Academic Press, London.
TRIPATHI, R. D., SRIVASTAVA, H. S., and DIXIT, S. N. 1980. Regulation of nitrate reductase, soluble and protein nitrogen by lawsone in Helminthosporium oryzae Breda de Haan. Experientia 36:960–961.
VLADIMIRTSEV, I. F., and STROMBERG, A. G. 1957. Polarographic study of 2,3-derivatives of 1,4-naphthoquinones. J. Gen. Chem. USSR (Engl. transl.) 27:1110–1123.
WEISSENBERG, M., KLEIN, M., MEISNER, J., and ASCHER, K. R. S. 1986. Larval growth inhibition of the spiny bollworm, Earias insulana, by some steroidal secondary plant compounds. Entomol. Exp. Appl. 42:213–217.
ZUMAN, P. 1962. Quantitative treatments of substituent effects in polarography. IV. Linear free energy relationships in quinoid series. Collect. Czech. Chem. Commun. 27:2035–2057.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Weissenberg, M., Meisner, J., Klein, M. et al. Effect of Substituent and Ring Changes in Naturally Occurring Naphthoquinones on the Feeding Response of Larvae of the Mexican Bean Beetle, Epilachna varivestis . J Chem Ecol 23, 3–18 (1997). https://doi.org/10.1023/B:JOEC.0000006342.51040.90
Issue Date:
DOI: https://doi.org/10.1023/B:JOEC.0000006342.51040.90