Abstract
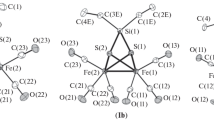
Two Fe–Ta containing sulfido complexes were prepared by the reaction of the metal halide salts with bis-trimethylsilylsulfide in the presence of PMe3. The complexes demonstrate that coordination chemistry with iron sulfides can give access to a range of heterometallic complexes. In [Cl(Me3P)Ta(μ 2-S)2(μ 3-S)Fe(PMe3)2]2 the two [Cl(Me3P)Ta] units are arranged around one central Fe2(μ 2-S)2 unit. In [(Me3P)4(MeCN)2FeII]2+[(Me3P)2TaIVFeII 3(μ 3-S)4Br4]2− a [TaFe3S4]2+ cuboidal arrangement was observed. The complex salt forms a polymeric structure in the solid-state with weak H-bonds between the ions. The [(Me3P)2TaIVFeII 3(μ 3-S)4Br4]2− ion was characterised by magnetic measurements showing strong antiferromagnetic interactions between the metal centres.
Similar content being viewed by others
REFERENCES
H.-C. Zhou and R. H. Holm (2003). Inorg. Chem. 42, 11 and references therein.
(a) F. Osterloh, B. M. Segal, C. Achim, and R. H. Holm (2000). Inorg. Chem. 39, 980. (b) C. Hauser, E. Bill, and R. H. Holm (2002). Inorg. Chem. 41, 1615. (c) K. D. Demadis, C. F. Campana, and D. Coucouvanis (1995). J. Am. Chem. Soc. 117, 7832. (d) J. Han, M. Koutmos, S. Al-Ahmat, and D. Coucouvanis (2001). Inorg. Chem. 40, 5985. (e) K. K. P. Srivastava, K. K. Surerus, R. C. Conover, M. K. Johnson, J.-B. Park, M. W. W. Adams, and E. Münck (1993). Inorg.Chem. 32, 927 and references therein.
For nitrogenase work see, e.g., B. J. Hales, E. E. Case, J. E. Morningstar, M. F. Dzeda, and L. A. Mauterer (1986). Biochemistry 25, 7251; for catalytic applications see, e.g., C. Bianchini and A. Meli (1996). J. Chem. Soc., Dalton Trans. 801; for investigations of optical properties see, e.g., S. Shi, W. Ji, S. H. Tang, J. P. Lang, and X. Q. Xin (1994). J. Am. Chem. Soc. 116, 3615.
See R. Pätow and D. Fenske (2002). Z. Anorg. Allg. Chem. 628, 1279 and 2790 for examples of coinage metal thiotantalates.
S. C. Lee and R. H. Holm (1990). J. Am. Chem. Soc. 112, 9654.
(a) H. Schmid and H. Ruf (1963). Z. Anorg. Allg. Chem. 321, 270. (b) M. L. Luetkens, A. P. Sattelberger, H. H. Murray, J. D. Basil, and J. P. Fackler (1989). Inorg. Synth. 26, 7.
G. M. Sheldrick, SHELXTL-97 University of Göttingen, 1997).
E. A. Boudreaux and L. N. Mulay (eds.), Theory and Applications of Molecular Paramagnetism (Wiley, New York, 1976).
D. Sellmann, M. Geck, F. Knoch, G. Ritter, and J. Dengler (1991). J. Am. Chem. Soc. 113, 3819.
S. M. Malinak, K. D. Demadis, and D. Coucouvanis (1995). J. Am. Chem. Soc. 117, 3126.
For a recent classification of H-bonds see, e.g., C. Giacovazzo, H. L. Monaco, G. Artioli, D. Viterbo, G. Ferraris, G. Gilli, G. Zanotti, and M. Catti, in C. Giacovazzo (ed.), Fundamentals of Crystallography (Oxford University Press, Oxford, 2002), p. 592.
J. E. Barclay, A. Hills, D. L. Hughes, and G. J. Leigh (1988). J. Chem. Soc., Dalton Trans. 2871.
S. J. Yoo, H. C. Angove, B. K. Burgess, M. P. Hendrich, and E. Münck (1999). J. Am. Chem. Soc. 121, 2534.
Y. Sanakis, S. J. Yoo, F. Osterloh, R. H. Holm, and E. Münck (2002). Inorg. Chem. 41, 7081.
R. L. Carlin, Magnetochemistry (Springer, Berlin, 1986).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Clérac, R., Fenske, D., Issac, I. et al. Syntheses and Structures of Heterometallic Fe–Ta Chalcogenido Clusters. Journal of Cluster Science 15, 189–198 (2004). https://doi.org/10.1023/B:JOCL.0000027402.38129.5b
Issue Date:
DOI: https://doi.org/10.1023/B:JOCL.0000027402.38129.5b