Abstract
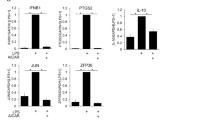
Gold sodium thiomalate (GST), chloroquine (CQ), and metho- trexate have been widely used in the therapy of rheumatoid arthritis and other inflammatory conditions. Using the human monocytic cell line THP-1 we have analyzed effects of these drugs on cytokine production and intracellular signaling. GST and CQ were equally effective in reducing lipopolysaccharide (LPS)-induced IL-1β release while CQ was a more effective inhibitor of TNF-α production than GST. Methotrexate did not affect production of these cytokines. CQ reduced IL-1β mRNA expression and strongly inhibited phosphorylation of mitogen-activated protein kinase (MAPK) p38, and to a lesser extent c-Jun N-terminal kinase and extracellular signal-regulated kinase 1/2. In contrast, GST did not affect cytokine mRNA expression or MAPK activation. However, GST selectively inhibited the activity of the interleukin-1 converting enzyme (ICE)/caspase-1. These data demonstrate that CQ inhibits IL-1β release from monocytes by interfering with pretranscriptional signaling and TNF-α release by posttranslational events whereas GST downregulates IL-1β secretion by interfering with posttranslational IL-1β processing.
Similar content being viewed by others
References
Danning CL, Boumpas DT: Commonly used disease-modifying antirheumatic drugs in the treatment of inflammatory arthritis: An update on mechanisms of action. Clin Exp Rheumatol 16:595–604, 1998
Walz DT, DiMartino MJ, Griswold DE: Comparative pharmacology and biological effects of different gold compounds. J Rheumatol 8 (Suppl):54–60, 1982
Remvig L, Enk C, Bligaard N: Effect of auranofin and sodium aurothiomalate on interleukin-1 production from human monocytes in vitro. Scand J Rheumatol 17:255–262, 1988
Danis VA, Kulesz AJ, Kelly DE, Nelson DS, Brooks PM: The effect of gold sodium thiomalate and auranofin on lipopolysaccharide-induced interleukin-1 production by blood monocytes in vitro: Variation in healthy subjects and patients with arthritis. Clin Exp Immunol 79:335–340, 1990
Chang DM, Baptiste P, and Schur PH: The effect of antirheumatic drugs on interleukin 1 (IL-1) activity and IL-1 and IL-1 inhibitor production by human monocytes. J Rheumatol 17:1148–1157, 1990
Crilly A, Madhok R, Watson J, Capell HA, Sturrock RD: Production of interleukin-6 by monocytes isolated from rheumatoid arthritis patients receiving second-line drug therapy. Br J Rheumatol 33:821–825, 1994
Evans GF, Zuckerman SH: Pharmacologic modulation of TNF production by endotoxin stimulated macrophages: In vitro and in vivo effects of auranofin and other chrysotherapeutic compounds. Agents Actions 26:329–334, 1989
Seitz M, Dewald B, Ceska M, Gerber N, Baggiolini M: Interleukin-8 in inflammatory rheumatic diseases: Synovial fluid levels, relation to rheumatoid factors, production by mononuclear cells, and effects of gold sodium thiomalate and methotrexate. Rheumatol Int 12:159–164, 1992
Loetscher P, Dewald B, Baggiolini M, Seitz M: Monocyte chemoattractant protein 1 and interleukin 8 production by rheumatoid synoviocytes. Effects of anti-rheumatic drugs. Cytokine 6:162–170, 1994
Sfikakis PP, Souliotis VL, Panayiotidis PP: Suppression of interleukin-2 and interleukin-2 receptor biosynthesis by gold compounds in in vitro activated human peripheral blood mononuclear cells. Arthritis Rheum 36:208–212, 1993
Hirohata S, Yanagida T, Hashimoto H, Tomita T, Ochi T, Nakamura H, Yoshino S: Differential influences of gold sodium thiomalate and bucillamine on the generation of CD14+ monocyte-lineage cells from bone marrow of rheumatoid arthritis patients. Clin Immunol Immunopathol 84:290–295, 1997
Kirkham BW, Navarro FJ, Corkill MM, Panayi GS: In vivo analysis of disease modifying drug therapy activity in rheumatoid arthritis by sequential immunohistological analysis of synovial membrane interleukin 1 beta. J Rheumatol 21:1615–1619, 1994
Kerimova AA, Atalay M, Yusifov EY, Kuprin SP, and Kerimov TM: Antioxidant enzymes; possible mechanism of gold compound treatment in rheumatoid arthritis. Pathophysiol 7:209, 2000
Yamashita M, Ichinowatari G, Yamaki K, Ohuchi K: Inhibition by auranofin of the production of prostaglandin E2 and nitric oxide in rat peritoneal macrophages. Eur J Pharmacol 368:251–258, 1999
Handel ML, Watts CK, deFazio A, Day RO, Sutherland RL: Inhibition of AP-1 binding and transcription by gold and selenium involving conserved cysteine residues in Jun and Fos. Proc Natl Acad Sci USA 92:4497–4501, 1995
Jeon KI, Jeong JY, Jue DM: Thiol-reactive metal compounds inhibit NF-kappa B activation by blocking I kappa B kinase. J Immunol 164:5981–5989, 2000
Yang JP, Merin JP, Nakano T, Kato T, Kitade Y, Okamoto T: Inhibition of the DNA-binding activity of NF-kappa B by gold compounds in vitro. FEBS Lett 361:89–96, 1995
Kataoka K, Handa H, Nishizawa M: Induction of cellular antioxidative stress genes through heterodimeric transcription factor Nrf2/small Maf by antirheumatic gold(I) compounds. J Biol Chem 276:34074–34081, 2001
Hashimoto K, Whitehurst CE, Matsubara T, Hirohata K, Lipsky PE: Immunomodulatory effects of therapeutic gold compounds. Gold sodium thiomalate inhibits the activity of T cell protein kinase C. J Clin Invest 89:1839–1848, 1992
Wong K, Parente J, Prasad KV, Ng D: Auranofin modulated cytoplasmic free calcium in neutrophils by mobilizing intracellular calcium and inhibiting protein kinase. J Biol Chem 265:21454–21461, 1990
Mahoney CW, Hensey CE, Azzi A: Auranofin, gold thiomalate, and gold thioglucose inhibit protein kinase C. Biochem Pharmacol 38:3383–3386, 1989
MacIntyre AC, Cutler DJ: Role of lysosomes in hepatic accumulation of chloroquine. J Pharm Sci 77:196–199, 1988
Gonzalez-Noriega A, Grubb JH, Talkad V, Sly WS: Chloroquine inhibits lysosomal enzyme pinocytosis and enhances lysosomal enzyme secretion by impairing receptor recycling. J Cell Biol 85:839–852, 1980
Karres I, Kremer JP, Dietl I, Steckholzer U, Jochum M, Ertel W: Chloroquine inhibits proinflammatory cytokine release into human whole blood. Am J Physiol 274:R1058-R1064, 1998
Zhu X, Ertel W, Ayala A, Morrison MH, Perrin MM, Chaudry, IH: Chloroquine inhibits macrophage tumour necrosis factor-alpha mRNA transcription. Immunology 80:122–126, 1993
Weber SM, Levitz SM: Chloroquine interferes with lipopolysaccharide-induced TNF-alpha gene expression by a nonlysosomotropic mechanism. J Immunol 165:1534–1540, 2000
Seitz M: Molecular and cellular effects of methotrexate. Curr Opin Rheumatol 11:226–232, 1999
Miller DK, Ayala JM, Egger LA, Raju SM, Yamin TT, Ding GJ, Gaffney EP, Howard AD, Palyha OC, Rolando AM, Salley JP, Jhornberry NA, Xeidner JR, Xilliams JH, Chapman KT, Jackson J, Kostura MJ, Limjuco G, Molineaux SM, Mumford RA, Calayacy JR: Purification and characterization of active human interleukin-1 beta-converting enzyme from THP.1 monocytic cells. J Biol Chem 268:18062–18069, 1993
Nicholson DW, Ali A, Thornberry NA, Vaillancourt JP, Ding CK, Gallant M, Gareau Y, Griffin PR, Labelle M, Lazebnik YA, et al.: Identification and inhibition of the ICE/CED-3 protease necessary for mammalian apoptosis. Nature 376:37–43, 1995
Han J, Lee JD, Bibbs L, Ulevitch RJ: A MAP kinase targeted by endotoxin and hyperosmolarity in mammalian cells. Science 265:808–811, 1994
Lee JC, Laydon JT, McDonnell PC, Gallagher TF, Kumar S, Green D, McNulty D, Blumenthal MJ, Heys JR, Landvatter SW, et al. A protein kinase involved in the regulation of inflammatory cytokine biosynthesis. Nature 372:739–746, 1994
Swantek JL, Cobb MH, Geppert TD: Jun N-terminal kinase/stress-activated protein kinase (JNK/SAPK) is required for lipopolysaccharide stimulation of tumor necrosis factor alpha (TNF-alpha) translation: glucocorticoids inhibit TNF-alpha translation by blocking JNK/SAPK. Mol Cell Biol 17:6274–6282, 1997
Kostura MJ, Tocci MJ, Limjuco G, Chin J, Cameron P, Hillman AG, Chartrain NA, Schmidt JA: Identification of a monocyte specific pre-interleukin 1 beta convertase activity. Proc Natl Acad Sci USA 86:5227–5231, 1989
Fantuzzi G, Dinarello CA: Interleukin-18 and interleukin-1 beta: Two cytokine substrates for ICE (caspase-1). J Clin Immunol 19:1–11, 1999
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Seitz, M., Valbracht, J., Quach, J. et al. Gold Sodium Thiomalate and Chloroquine Inhibit Cytokine Production in Monocytic THP-1 Cells Through Distinct Transcriptional and Posttranslational Mechanisms. J Clin Immunol 23, 477–484 (2003). https://doi.org/10.1023/B:JOCI.0000010424.41475.17
Issue Date:
DOI: https://doi.org/10.1023/B:JOCI.0000010424.41475.17