Abstract
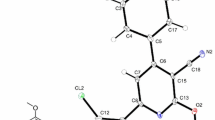
The reaction of dichloromaleic anhydride with 1,8-diaminonaphthalene in refluxing toluene or 1,2-dichloroethane produces the new heterocyclic compound 8,9-dichloropyrrolo[1,2-a]perimidin-10-one in low yields. 8,9-Dichloropyrrolo[1,2-a]perimidin-10-one has been isolated by column chromatography and characterized in solution by IR, 1H NMR, and UV/vis spectroscopies. The solid-state structure was unequivocally established by single-crystal X-ray diffraction analysis. 8,9-Dichloropyrrolo[1,2-a]perimidin-10-one crystallizes in the monoclinic space group P2 1/c, a = 7.475(1)Å, b = 10.650(2)Å, c = 14.468(2)Å, β = 94.478(2)°, V = 1148.3(3) Å3, Z = 4, and d calc = 1.672 Mg/m3; R = 0.0289, R w = 0.0762 for 1644 reflections with I > 2σ(I). The nature of the HOMO and LUMO in 8,9-dichloropyrrolo[1,2-a]perimidin-10-one has been determined by extended Hückel molecular orbital calculations, and these data are discussed relative to the cyclic voltammetric data obtained at a platinum electrode.
Similar content being viewed by others
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Watson, W.H., Chen, T. & Richmond, M.G. Reaction of dichloromaleic anhydride with 1,8-diaminonaphthalene: Synthesis and X-ray diffraction structure of 8,9-dichloropyrrolo[1,2-a]perimidin-10-one. Journal of Chemical Crystallography 34, 697–703 (2004). https://doi.org/10.1023/B:JOCC.0000047646.33079.d2
Issue Date:
DOI: https://doi.org/10.1023/B:JOCC.0000047646.33079.d2