Abstract
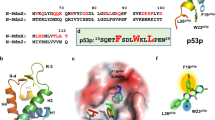
MDM2 is a regulator of cell growth processes that acts by binding to the tumor suppressor protein p53 and ultimately restraining its activity. While inactivation of p53 by mutation is commonly observed in human cancers, a substantial percentage of tumors express wild type p53. In many of these cases, MDM2 is overexpressed, and it is believed that suppression of MDM2 activity could yield therapeutic benefits. Therefore, we have been focusing on the p53-MDM2 interaction as the basis of a drug discovery program and have been able to develop a series of small molecule inhibitors. We herein report a high resolution NMR structure of a complex between the p53-binding domain of MDM2 and one of these inhibitors. The form of MDM2 utilized was an engineered hybrid between the human and Xenopus sequences, which provided a favorable combination of relevancy and stability. The inhibitor is found to bind in the same site as does a highly potent peptide fragment of p53. The inhibitor is able to successfully mimic the peptide by duplicating interactions in three subpockets normally made by amino acid sidechains, and by utilizing a scaffold that presents substituents with rigidity and spatial orientation comparable to that provided by the alpha helical backbone of the peptide. The structure also suggests opportunities for modifying the inhibitor to increase its potency.
Similar content being viewed by others
References
Arkin, M.R., Randal, M., DeLano, W.L., Hyde, J., Luong, T.N., Oslob, J.D., Raphael, D.R., Taylor, L., Wang, J., McDowell, R.S., Wells, J.A. and Braisted, A.C. (2003) Proc. Natl. Acad. Sci. USA, 100, 1603–1608.
Asada, S., Choi, Y. and Uesugi, M. (2003) J. Am. Chem. Soc., 125, 4992–4993.
Bax, A., Clore, G.M. and Gronenborn, A.M. (1990) J. Magn. Reson., 88, 425–431.
Berg, T. (2003) Agnew. Chem. Int. Ed., 42, 2462–2481.
Berg, T., Cohen, S.B., Desharnais, J., Sonderegger, C., Maslyar, D.J., Goldberg, J., Boger, D.L. and Vogt, P.K. (2002) Proc. Natl. Acad. Sci. USA, 99, 3830–3835.
Bohacek, R.S., Dalgarno, D.C., Hatada, M., Jacobsen, V.A., Lynch, B.A., Macek, K.J., Merry, T., Metcalf, C.A., Narula, S.S., Saw-yer, T.K., Shakespeare, W.C., Violette, S.M. and Weigele, M. (2001) J. Med. Chem., 44, 660–663.
Braisted, A.C., Oslob, J.D., Delano, W.L., Hyde, J., McDowell, R.S., Waal, N., Yu, C., Arkin, M.R. and Raimundo, B.C. (2003) J. Am. Chem. Soc., 125, 3714–3715.
Brinkmann, U., Mattes, R.E., Buckel, P. (1989) Gene 85,109–114.
Bueso-Ramos, C.E., Yun, Y., deLeon, E., McCowm, P., Stass, S. and Albitar, M. (1993) Blood, 82, 2617–2623.
Carter, P.H., Scherle, P.A., Muckelbauer, J.A., Voss, M.E., Liu, R.-Q., Thompson, L.A., Tebben, A.J., Solomon, K.A., Lo, Y.C., Li, Z., Strzemienski, P., Yang, G., Falahatpisheh, N., Xu, M., Wu, Z., Farrow, N.A., Ramnarayan, K., Wang, J., Rideout, D., Yalamoori, V., Domaille, P., Underwood, D.J., Trzaskos, J.M., Freidman, S.M., Newton, R.C. and Decicco, C.P. (2001) Proc. Natl. Acad. Sci. USA, 98, 11879–11884.
Chen, J., Marechal, V. and Levine, A.J. (1993) Mol. Cell. Biol., 13, 4107–4114.
Chen, L., Tilley, J.W., Trilles, R.V., Yun, W., Fry, D., Cook, C., Rowan, K., Schwinge, V. and Campbell, R. (2002) Bioorg. Med. Chem. Lett., 12, 137–141.
Clackson, T. and Wells, J.A. (1995) Science, 267, 383–386.
Clore, G.M. and Gronenborn, A.M. (1994) In Methods in Enzymo-logy, Vol. 239: Nuclear Magnetic Resonance, Part C, James, T.L. and Oppenheimer, N.J. (Eds.), Academic Press, San Diego, pp. 349–363.
Cornilescu, G., Delaglio, F. and Bax, A. (1999) J. Biomol. NMR, 13, 289–302.
Emerson, S.D., Madison, V.S., Palermo, R.E., Waugh, D.S., Scheffler, J.E., Tsao, K.-L., Kiefer, S.E., Liu, S.P. and Fry, D.C. (1995) Biochemistry, 34, 6911–6918.
Emerson, S.D., Palermo, R., Liu, C.-M., Tilley, J.W., Chen, L., Danho, W., Madison, V.S., Greeley, D.N., Ju, G. and Fry, D.C. (2003) Protein Sci., 12, 811–822.
Gadek, T.R., Burdick, D.J., McDowell, R.S., Stanley, M.S., Marsters, J.C., Paris, K.J., Oare, D.A., Reynolds, M.E., Lad-ner, C., Zioncheck, K.A., Lee, W.P., Gribling, P., Dennis, M.S., Skelton, N.J., Tumas, D.B., Clark, K.R., Keating, S.M., Beresini, M.H., Tilley, J.W., Presta, L.G. and Bodary, S.C. (2002) Science, 295, 1086–1089.
Garcia-Echeverria, C., Chene, P., Blommers, M.J.J. and Furet, P. (2000) J. Med. Chem., 43, 3205–3208.
Haupt, Y., Maya, R., Kazaz, A. and Oren, M. (1997) Nature, 387, 296–299.
Higuchi, R., Krummel, B. and Saiki, R.K. (1988) Nucl. Acids Res., 16, 7351–7367.
Ho, S.N., Hunt, H.D., Horton, R.M., Pullen, J.K. and Pease, L.R. (1989) Gene, 77, 51–59.
Hollstein, M., Sidransky, D., Vogelstein, B. and Harris, C.C. (1991) Science, 253, 49–53.
Ikura, M. and Bax, A. (1992) J. Am. Chem. Soc., 114, 2433–2440.
Jennerwein, M., Wappes, B., Gust, R., Schonenberger, H., Engel, J., Seeber, S. and Osieka, R. (1988) J. Can. Res. Clin. Oncol., 114, 347–358.
Johnson, B.A. and Blevins, R.A. (1994) J. Biomol. NMR, 4, 603–614.
Kallen, J., Weizenbach, K., Ramage, P., Geyl, D., Kriwacki, R., Legge, G., Cottens, S., Weitz-Schmidt, G. and Hommel, U. (1999) J. Mol. Biol., 292, 1–9.
Kay, L.E., Keifer, P. and Saarinen, T. (1992) J. Am. Chem. Soc., 114, 10663–10665.
Ku, T.W., Ali, F.E., Barton, L.S., Bean, J.W., Bondinell, W.E., Burgess, J.L., Callahan, J.F., Calvo, R.R., Chen, L., Eggleston, D.S., Gleason, J.G., Huffman, W.F., Hwang, S.M., Jakas, D.R., Karash, C.B., Keenan, R.M., Kopple, K.D., Miller, W.H., New-lander, K.A., Nichols, A., Parker, M.F., Peishoff, C.E., Samanen, J.M., Uzinskas, I. and Venslavsky, J.W. (1993) J. Am. Chem. Soc., 115, 8861–8862.
Kubbutat, M.H.G., Jones, S.N. and Vousden, K.H. (1997) Nature, 387, 299–303.
Kussie, P.H., Gorina, S., Marechal, V., Elenbaas, B., Moreau, J., Levine, A.J. and Pavletich, N.P. (1996) Science, 274, 948–953.
Laskowski, R.A., Rullmann, J.A.C., MacArthur, M.W., Kaptein, R. and Thornton, J.M. (1996) J. Biomol. NMR, 8, 477–496.
Last-Barney, K., Davidson, W., Cardozo, M., Frye, L.L., Grygon, C.A., Hopkins, J.L., Jeanfavre, D.D., Pav, S., Qian, C., Steven-son, J.M., Tong, L., Zindell, R. and Kelly, T.A. (2001) J. Am. Chem. Soc., 123, 5643–5650.
Levine, A.J. (1997) Cell, 88, 323–331.
Liu, G., Huth, J.R., Olejniczak, E.T., Mendoza, R., DeVries, P., Leitza, S., Reilly, E.B., Okasinski, G.F., Fesik, S.W. and von Geldern, T.W. (2001) J. Med. Chem., 44, 1202–1210.
Lunney, E.A., Para, K.S., Rubin, J.R., Humblet, C., Fergus, J.H., Marks, J.S. and Sawyer, T.K. (1997) J. Am. Chem. Soc., 119, 12471–12476.
Marchetti, A. (1995) J. Pathol., 173, 31–38.
McCoy, M.A. and Mueller, L. (1992) J. Am. Chem. Soc., 114, 2108–2112.
McDowell, R.S., Blackburn, B.K., Gadek, T.R., McGee, L.R., Rawson, T., Reynolds, M.E., Robarge, K.D., Somers, T.C., Thor-sett, E.D., Tischler, M., Webb, R.R. and Venuti, M.C. (1994) J. Am. Chem. Soc., 116, 5077–5083.
McMillan, K., Adler, M., Auld, D.S., Baldwin, J.J., Blasko, E., Browne, L.J., Chelsky, D., Davey, D., Dolle, R.E., Eagen, K.A., Erickson, S., Feldman, R.I., Glaser, C.B., Mallari, C., Morris-sey, M.M., Ohlmeyer, M.H.J., Pan, G., Parkinson, J.F., Phillips, G.B., Polokoff, M.A., Sigal, N.H., Vergona, R., Whitlow, M., Young, T.A. and Devlin, J.J. (2000) Proc. Natl. Acad. Sci. USA, 97, 1506–1511.
Midgley, C.A. and Lane, D.P. (1997) Oncogene, 15, 1179–1189.
Muhandiram, D.R. and Kay, L.E. (1994) J. Magn. Reson., 103, 203–216.
Nagayama, K. (1986) J. Magn. Reson., 66, 240–249.
Nilges, M. Clore, G.M. and Gronenborn, A.M. (1988a) FEBS Lett., 239, 129–136.
Nilges, M., Gronenborn, A.M., Brunger, A.T. and Clore, G.M. (1988b) Protein Eng., 2, 27–38.
Oliner, J.D., Kinzler, K.W., Meltzer, P.S., George, D. and Vogel-stein, B. (1992) Nature, 358, 80–83.
Orner, B.P., Ernst, J.T. and Hamilton, A.D. (2001) J. Am. Chem. Soc., 123, 5382–5383.
Picksley, S.M., Vojtesek, B., Sparks, A. and Lane, D.P. (1994) Oncogene, 9, 2523–2529.
Proudfoot, J. R., Betageri, R., Cardozo, M., Gilmore, T.A., Glynn, S., Hickey, E.R., Jakes, S., Kabcenell, A., Kirrane, T.M., Tibolla, A.K., Lukas, S., Patel, U.R., Sharma, R., Yazdanian, M. and Moss, N. (2001) J. Med. Chem., 44, 2421–2431.
Reifenberger, G., Lu, L., Ichimura, K., Schmidt, E.E. and Collins, V.P. (1993) Cancer Res., 53, 2736–2739.
Rutledge, S.E., Chin, J.W. and Schepartz, A. (2002) Curr. Opin. Chem. Biol., 6, 479–485.
Shaka, A.J., Lee, C.J. and Pines, A. (1988) J. Magn. Reson., 77, 274–293.
Shibagaki, I., Tanaka, H., Shimada, Y., Wagata, T., Ikenaga, M., Imamura, M. and Ishizaki, K. (1995) Clin. Cancer Res., 1, 769–773.
Smith, A.B., Hirshmann, R., Pasternak, A., Yao, W., Sprengeler, P.A., Holloway, M.K., Kuo, L.C., Chen, Z., Darke, P.L. and Schleif, W.A. (1997) J. Med. Chem., 40, 2440–2444.
Stoll, R., Renner, C., Hansen, S., Palme, S., Kelin, C., Belling, A., Zeslawski, W., Kamionka, M., Rehm, T., Muhlhahn, P., Schumacher, R., Hesse, F., Kaluza, B., Voelter, W., Engh, R. and Holak, T. (2001) Biochemistry, 40, 336–344.
Stonehouse, J., Shaw, G.L., Keeler, J. and Laue, E.D. (1994) J. Magn. Reson., 107, 178–184.
Tilley, J.W., Chen, L., Fry, D.C., Emerson, S.D., Powers, G.D., Biondi, D., Varnell, T., Trilles, R., Guthrie, R., Mennona, F., Kaplan, G., LeMahieu, R.A., Carson, M., Han, R.-J., Liu, C.-M., Palermo, R. and Ju, G. (1997) J. Am. Chem. Soc., 119, 7589–7590.
Toogood, P.L. (2002) J. Med. Chem., 45, 1543–1558.
Vogtle, F. and Goldschmitt, E. (1976) Chem. Ber., 109, 1–40.
Zheleva, D.I., Lane, D.P. and Fischer, P.M. (2003) Mini Rev. Med. Chem., 3, 257–270.
Zhu, Y.-F., Wang, X.-C., Connors, P., Wilcoxen, K., Gao, Y., Gross, R., Strack, N., Gross, T., McCarthy, J.R., Xie, Q., Ling, N. and Chen, C. (2003) Bioorg. Med. Chem. Lett., 13, 1931–1934.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Fry, D.C., Emerson, S.D., Palme, S. et al. NMR structure of a complex between MDM2 and a small molecule inhibitor. J Biomol NMR 30, 163–173 (2004). https://doi.org/10.1023/B:JNMR.0000048856.84603.9b
Issue Date:
DOI: https://doi.org/10.1023/B:JNMR.0000048856.84603.9b